CRS alternatives
- Cpt.Frederickson
- Hollow Legs
- Posts: 454
- Joined: Wed Jul 04, 2012 7:54 pm
- Location: BIAB in the Shed, Maidstone, Kent
Re: CRS alternatives
Awesome! Last time I checked they were doing a minimum of 25ltrs! I would like to see a little further info so may have to email Paul for some details. Have you used it yet and if so how was it?
Think this may be another thing for the shopping list...
Think this may be another thing for the shopping list...
The Hand of Doom Brewery and Meadery
Fermenting -
Conditioning - Meads - Raspberry Melomel yeast test, Vanilla Cinnamon Metheglyn, Orange Melomel.
Drinking - Youngs AAA Kit; Leatherwood Traditional Mead, Cyser, Ginger Metheglyn.
Planning - Some kits until I can get back to AG, then a hoppy porter, Jim's ESB, some American Red.
Fermenting -
Conditioning - Meads - Raspberry Melomel yeast test, Vanilla Cinnamon Metheglyn, Orange Melomel.
Drinking - Youngs AAA Kit; Leatherwood Traditional Mead, Cyser, Ginger Metheglyn.
Planning - Some kits until I can get back to AG, then a hoppy porter, Jim's ESB, some American Red.
Re: CRS alternatives
They only put it up this afternoon on the Homebrew site. Available in 250ml and 1L. I've ordered some, but not received it yet. Eager to try it.Cpt.Frederickson wrote:Awesome! Last time I checked they were doing a minimum of 25ltrs! I would like to see a little further info so may have to email Paul for some details. Have you used it yet and if so how was it?
Think this may be another thing for the shopping list...
- Eric
- Even further under the Table
- Posts: 2879
- Joined: Fri Mar 13, 2009 1:18 am
- Location: Sunderland.
Re: CRS alternatives
Hi Chastuck,chastuck wrote:They only put it up this afternoon on the Homebrew site. Available in 250ml and 1L. I've ordered some, but not received it yet. Eager to try it.Cpt.Frederickson wrote:Awesome! Last time I checked they were doing a minimum of 25ltrs! I would like to see a little further info so may have to email Paul for some details. Have you used it yet and if so how was it?
Think this may be another thing for the shopping list...
Can I ask if you got the sulphuric acid and what you've found?
Without patience, life becomes difficult and the sooner it's finished, the better.
Re: CRS alternatives
Yes I got the acid and it seems just fine. I haven't used in a brew yet, but an experiment I did on tap water shows it reduces the alkalinity just a little bit higher than Murphy's said it will do. Murphy’s is 25% w/w sulphuric acid, which I reckon is equivalent to 3M. Murphy say at 0.34ml per litre this will contribute about 100ppm of sulphate and reduce alkalinity by about 100ppm. My alkalinity reduction was nearer 120.Eric wrote:Hi Chastuck,chastuck wrote:They only put it up this afternoon on the Homebrew site. Available in 250ml and 1L. I've ordered some, but not received it yet. Eager to try it.Cpt.Frederickson wrote:Awesome! Last time I checked they were doing a minimum of 25ltrs! I would like to see a little further info so may have to email Paul for some details. Have you used it yet and if so how was it?
Think this may be another thing for the shopping list...
Can I ask if you got the sulphuric acid and what you've found?
I bought so many half-price kits from Wilkos in their recent sale that I reckon I will not return to all-grain brewing much before Christmas, so will not know actual benefit in brew water for a while yet.
- Eric
- Even further under the Table
- Posts: 2879
- Joined: Fri Mar 13, 2009 1:18 am
- Location: Sunderland.
Re: CRS alternatives
Thanks for this. Mine is also marked 25% w/w but their data sheet at http://www.murphyandson.co.uk/datasheet ... 025%25.pdf says differently and I got a bit of a shock when I put the bottle on the scales.chastuck wrote: Yes I got the acid and it seems just fine. I haven't used in a brew yet, but an experiment I did on tap water shows it reduces the alkalinity just a little bit higher than Murphy's said it will do. Murphy’s is 25% w/w sulphuric acid, which I reckon is equivalent to 3M. Murphy say at 0.34ml per litre this will contribute about 100ppm of sulphate and reduce alkalinity by about 100ppm. My alkalinity reduction was nearer 120.
I bought so many half-price kits from Wilkos in their recent sale that I reckon I will not return to all-grain brewing much before Christmas, so will not know actual benefit in brew water for a while yet.
You've beaten me to my next question as I too found alkalinity reduction greater than their data, even more than you report.
Without patience, life becomes difficult and the sooner it's finished, the better.
Re: CRS alternatives
Their data sheet is incorrect, perhaps a mistype. A 25% H2SO4 (W/W) has 25gm H2SO4 per 100gm of solution. My experiments shows 50ml of Murphy 25% weighs 59gm. Therefore the density of Murphy H2SO4 = 1.180 kg/L. From standard density/percent and w/w charts, this equates to strength = 25 – 26%, which shows Murphy's is about right. (1L of 25% weighs 1180gm.)Eric wrote:Thanks for this. Mine is also marked 25% w/w but their data sheet at http://www.murphyandson.co.uk/datasheet ... 025%25.pdf says differently and I got a bit of a shock when I put the bottle on the scales.chastuck wrote: Yes I got the acid and it seems just fine. I haven't used in a brew yet, but an experiment I did on tap water shows it reduces the alkalinity just a little bit higher than Murphy's said it will do. Murphy’s is 25% w/w sulphuric acid, which I reckon is equivalent to 3M. Murphy say at 0.34ml per litre this will contribute about 100ppm of sulphate and reduce alkalinity by about 100ppm. My alkalinity reduction was nearer 120.
I bought so many half-price kits from Wilkos in their recent sale that I reckon I will not return to all-grain brewing much before Christmas, so will not know actual benefit in brew water for a while yet.
You've beaten me to my next question as I too found alkalinity reduction greater than their data, even more than you report.
- Eric
- Even further under the Table
- Posts: 2879
- Joined: Fri Mar 13, 2009 1:18 am
- Location: Sunderland.
Re: CRS alternatives
Yes, their data sheet is wrong, what I'm struggling to find is what, if any, is right.chastuck wrote:Their data sheet is incorrect, perhaps a mistype. A 25% H2SO4 (W/W) has 25gm H2SO4 per 100gm of solution. My experiments shows 50ml of Murphy 25% weighs 59gm. Therefore the density of Murphy H2SO4 = 1.180 kg/L. From standard density/percent and w/w charts, this equates to strength = 25 – 26%, which shows Murphy's is about right. (1L of 25% weighs 1180gm.)Eric wrote:Thanks for this. Mine is also marked 25% w/w but their data sheet at http://www.murphyandson.co.uk/datasheet ... 025%25.pdf says differently and I got a bit of a shock when I put the bottle on the scales.chastuck wrote: Yes I got the acid and it seems just fine. I haven't used in a brew yet, but an experiment I did on tap water shows it reduces the alkalinity just a little bit higher than Murphy's said it will do. Murphy’s is 25% w/w sulphuric acid, which I reckon is equivalent to 3M. Murphy say at 0.34ml per litre this will contribute about 100ppm of sulphate and reduce alkalinity by about 100ppm. My alkalinity reduction was nearer 120.
I bought so many half-price kits from Wilkos in their recent sale that I reckon I will not return to all-grain brewing much before Christmas, so will not know actual benefit in brew water for a while yet.
You've beaten me to my next question as I too found alkalinity reduction greater than their data, even more than you report.
My litre bottle weighed 1.417 kg and after allowing a reasonable amount for the bottle it would seem to be more dense than yours. It also seems to remove more alkalinity than yours. I'm only glad we test alkalinity after dosing.
Without patience, life becomes difficult and the sooner it's finished, the better.
-
MillmoorRon
Re: CRS alternatives
Been looking into water treatment and have a question regarding acid additions.nigelsch wrote:Hydro acid - 11.64N (can't remember percentage but normality is the important one)
- takes out 582ppm alk & adds 412ppm cl+ /ml/L
Sulphuric - 36N
- takes out 1800ppm alk & adds 1730 so4+ /ml/L
Nige
What happens when the carbonate levels approach zero and more acid is added?
- Aleman
- It's definitely Lock In Time
- Posts: 6132
- Joined: Sun Jun 03, 2007 11:56 am
- Location: Mashing In Blackpool, Lancashire, UK
Re: CRS alternatives
You remove all buffering capability of the mash liquor, and the mash pH 'crashes' to what may be an unacceptably low figure (Certainly outside of the range of 5.3 to 5.MillmoorRon wrote:Been looking into water treatment and have a question regarding acid additions.
What happens when the carbonate levels approach zero and more acid is added?
Fundamentally though it will still produce beer, but it may very well not be great beer. It is for this reason that the advice is generally given to add half to two thirds of the acid recommended, test the alkalinity again, and then add any additional acid to get the alkalinity where you want it.
For the sparge liquor,it will have little effect BUT it will lower the pH in the boil/fermentation and bottle, affecting the processes that take place there.
-
sladeywadey
- Hollow Legs
- Posts: 374
- Joined: Thu Dec 03, 2009 5:56 pm
Re: CRS alternatives
is there another source of Sulphuric now that Murphy's have stopped supplying the HB market?
-
Dave S
- Even further under the Table
- Posts: 2514
- Joined: Sun Apr 01, 2012 5:38 pm
- Location: Wirral, Merseyside
Re: CRS alternatives
APC Pure.sladeywadey wrote:is there another source of Sulphuric now that Murphy's have stopped supplying the HB market?
Best wishes
Dave
Dave
-
MillmoorRon
Re: CRS alternatives
So the carbonates react with the acid (changing the compostion of the water) but, while the buffering capability still exists, the pH does not decrease?Aleman wrote: You remove all buffering capability of the mash liquor, and the mash pH 'crashes' to what may be an unacceptably low figure (Certainly outside of the range of 5.3 to 5..
Once the buffers have been exhausted any additional acid addition will decrese the pH?
Is this correct?
My O-Level Chemistry is a distant memory!
- Aleman
- It's definitely Lock In Time
- Posts: 6132
- Joined: Sun Jun 03, 2007 11:56 am
- Location: Mashing In Blackpool, Lancashire, UK
Re: CRS alternatives
No, the pH does decrease gradually while you add the acid, at around pH 4.3 all the alkalinity is said to have been removed (not strictly true, but close enough).
Remember that the pH of the water is not important, what is important is the pH of the mash. Now the mash is a fairly complex self buffering environment, and what we are doing by reducing the alkalinity (the 'buffering element") of the liquor, is to change the pH at which the mash will buffer itself. Pale malt has a certain amount of buffering capability (from the phytin reaction), and crystal and roast malts provide more, which, coincidentally, is why dark beers can cope with a higher alkalinity than pale ones. Most mashes made with an average liquor and an 'average' grist will fall between 5.3 and 5.8, if they don't then we need to remove or add alkalinity. If we have no alkalinity in the mash then the pH will fall outside the optimal range for efficient enzyme activity.
Remember that the pH of the water is not important, what is important is the pH of the mash. Now the mash is a fairly complex self buffering environment, and what we are doing by reducing the alkalinity (the 'buffering element") of the liquor, is to change the pH at which the mash will buffer itself. Pale malt has a certain amount of buffering capability (from the phytin reaction), and crystal and roast malts provide more, which, coincidentally, is why dark beers can cope with a higher alkalinity than pale ones. Most mashes made with an average liquor and an 'average' grist will fall between 5.3 and 5.8, if they don't then we need to remove or add alkalinity. If we have no alkalinity in the mash then the pH will fall outside the optimal range for efficient enzyme activity.
- Eric
- Even further under the Table
- Posts: 2879
- Joined: Fri Mar 13, 2009 1:18 am
- Location: Sunderland.
Re: CRS alternatives
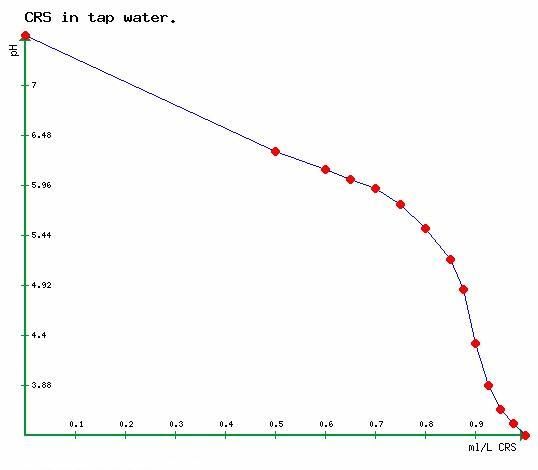
Below is a graph showing pH in a litre of a tapwater with alkalinity of approximately 160mg/l as CaCO3 when treated with CRS.MillmoorRon wrote:So the carbonates react with the acid (changing the compostion of the water) but, while the buffering capability still exists, the pH does not decrease?Aleman wrote: You remove all buffering capability of the mash liquor, and the mash pH 'crashes' to what may be an unacceptably low figure (Certainly outside of the range of 5.3 to 5..
Once the buffers have been exhausted any additional acid addition will decrese the pH?
Is this correct?
My O-Level Chemistry is a distant memory!
The graph is straight lined (for my ease) between the measurements shown in red.
As alkalinity approaches zero, the rate of reduction in pH increases for a given incremental acid addition, represented in the steepness of the curve. The point of steepest fall is the accepted end point of alkalinity which is somewhere (as already said) around pH 4.4.
Calcium in liquor reacts with malt (natural biological) to release hydrogen and lower mash pH which can be balanced by the influence of a suitable quantity of alkalinity. Liquor with very low amounts of calcium or none (malt supplies some calcium) release little hydrogen such that a small quantity of alkalinity or even none can result in an unacceptably high pH. Some calculators and some brewers too advocate adding excess acid to correct a too high mash pH with a low level of calcium, but I have misgivings about such advice. Acid malts are used to correct mash pH in beers made with low mineral content liquors, but these are the likes of lactic acid formed by bacterial fermentation, a natural product with flavour complimenting the style of beer. While any excess acid used to eliminate alkalinity would remain in the liquor to lower mash pH, I have concerns about doing this with a mineral acid like CRS. I also have concerns about using lactic acids in beers not of particular styles and tastes.
Without patience, life becomes difficult and the sooner it's finished, the better.


