Brun Water - RA & Bicarbonate goes minus, OK?
-
SiHoltye
Brun Water - RA & Bicarbonate goes minus, OK?
If I add the required amount of acid to bring mash ph to 5.4 (in my case I'm using phosphoric at 0.12mls/L so as not to increase sulphate) my bicarbonate level goes to -27.1
Is that OK/what impact does this minus figure have? I'm happy with the other numbers. Thanks
Is that OK/what impact does this minus figure have? I'm happy with the other numbers. Thanks
Last edited by SiHoltye on Sun May 25, 2014 7:38 pm, edited 1 time in total.
-
SiHoltye
Re: Brun Water - Bicarbonate goes minus, OK?
OK I didn't Google it.
Looks like nothing to worry about...
https://www.homebrewersassociation.org/ ... ic=15647.0
Looks like nothing to worry about...
https://www.homebrewersassociation.org/ ... ic=15647.0
-
paulg
Re: Brun Water - Bicarbonate goes minus, OK?
I had the same problem when I brewed a few brews back ,the bicarb went to -17 .I used 0.35 ml/L phosphoric for 40 litres water brew length 25 litres and epsom salt to get sulphate/chloride ratio .my mash ph was very low too 4.9-5.0
The resulting beer was very disapointing it seemed to lack in clarity of flavour .I then brewed the same recipe and adjusted the bicarbs to the recommended level for an amber beer and got mash ph down further using gypsum and calcium carbonate (my water is 220 ppm caco3).
this resulted in a much better flavoured beer.
I would be interested in your results as I still dont know if I miss measured my acid although bru n water predicted a negative bicarbonate but with a 5.4 mash ph
The resulting beer was very disapointing it seemed to lack in clarity of flavour .I then brewed the same recipe and adjusted the bicarbs to the recommended level for an amber beer and got mash ph down further using gypsum and calcium carbonate (my water is 220 ppm caco3).
this resulted in a much better flavoured beer.
I would be interested in your results as I still dont know if I miss measured my acid although bru n water predicted a negative bicarbonate but with a 5.4 mash ph
-
SiHoltye
Re: Brun Water - Bicarbonate goes minus, OK?
Here are my results from focusing on achieving mash ph mostly with acid and then sulphate/chloride adjustment for flavour. What sticks out are the minus RA and minus bicarbonate figures. Is there is a problem with this?
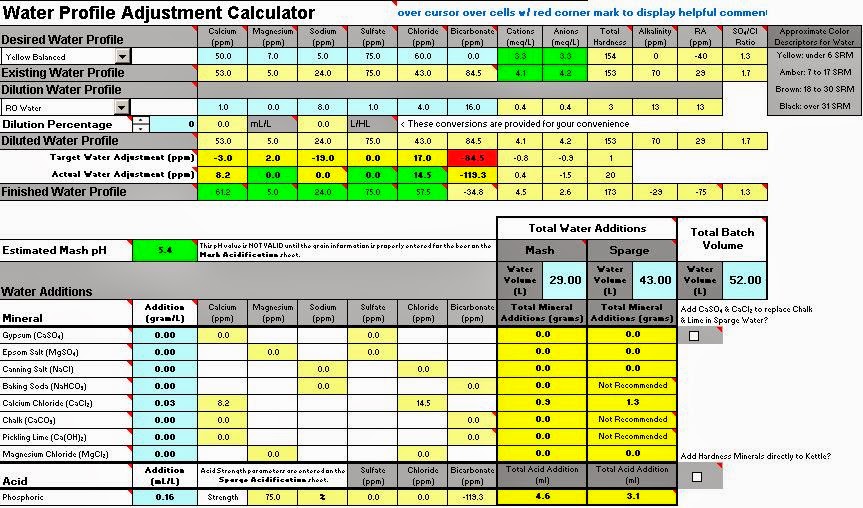

- mabrungard
- Piss Artist
- Posts: 250
- Joined: Sat Dec 15, 2012 3:17 pm
- Location: Indianapolis, Indiana
Re: Brun Water - RA & Bicarbonate goes minus, OK?
Yes, a negative RA is necessary for most pale beers. For example, an all Pils grist mashed in distilled water will produce a mash pH of around 5.7 to 5.8. That distilled water has a RA of zero. You have to add a bit of acid or acid malt to push the mash pH into the 5.2 to 5.4 range and avoid tannin extraction. That added acid will push the RA negative. Pale and amber beers are likely to require a negative RA and brown and black beers are likely to require RA in the positive range.
As always, its the mash pH target that matters. RA is not a target, it is a result.
As always, its the mash pH target that matters. RA is not a target, it is a result.
Martin B
Indianapolis, Indiana
BJCP National Judge
Foam Blowers of Indiana (FBI)
Brewing Water Information at: https://www.brunwater.com/
Like Bru'n Water on Facebook for occasional discussions on brewing water and Bru'n Water
https://www.facebook.com/pages/Brun-Wat ... =bookmarks
Indianapolis, Indiana
BJCP National Judge
Foam Blowers of Indiana (FBI)
Brewing Water Information at: https://www.brunwater.com/
Like Bru'n Water on Facebook for occasional discussions on brewing water and Bru'n Water
https://www.facebook.com/pages/Brun-Wat ... =bookmarks
-
SiHoltye
Re: Brun Water - RA & Bicarbonate goes minus, OK?
This is quite revolutionary for my water treatment then. Until now for pale beers such as this I've been adding CRS to achieve RA40 (0.19mls per litre) then adding 32.25g of Murphy's dry liquor salts per 52L brewlength (my standard). I can't wait to try these BnW additions.
- Eric
- Even further under the Table
- Posts: 2879
- Joined: Fri Mar 13, 2009 1:18 am
- Location: Sunderland.
Re: Brun Water - RA & Bicarbonate goes minus, OK?
Hi Si, Martin will give a fuller, better explanation than I, but residual alkalinity is not what you and I think of as alkalinity, that which is measurable by an instrument or after titration. It is a derivative, a hypothetical quantity found from experiments conducted by a German scientist on mashes with low ion water.SiHoltye wrote:This is quite revolutionary for my water treatment then. Until now for pale beers such as this I've been adding CRS to achieve RA40 (0.19mls per litre) then adding 32.25g of Murphy's dry liquor salts per 52L brewlength (my standard). I can't wait to try these BnW additions.
As you, I and every brewer in Britain can or has found, as mash water's alkalinity is reduced, so does the pH of that mash. However, if ion levels tend towards zero there seems to be an anomaly, when the mash pH rises again. These conditions probably don't exist in inhabited parts of UK as our rainfall tends to be, due to living on some rather narrow islands, dilute sea water with enough calcium, magnesium, sodium, chloride, etc and CO2 collected on route to avoid such circumstances. However, it exists in parts of Germany and other continental areas and Kohlbach produced an equation to calculate from specific components of a water to quantify what is called Residual Alkalinity, which when used, solved the apparent anomaly.
As you know, mash pH can and will be reduced by adding calcium and/or magnesium and as the opposite is also true, reducing those ions increases pH and when such ions tend to zero, the pH of that mash increases despite little or no alkalinity causing that to happen. So, if, and only if, you wish to mathematically predict mash pH, then the amounts of calcium and magnesium in the mash can be factored and used to adjust the actual alkalinity to what is called Residual Alkalinity.
There is dispute with Kohlbach's figures and his certainly don't work with my water, but the following is a rough and ready approximation of what was postulated by Kohlbach, as applied to my water.
My alkalinity is normally 250ppm CaCO3 and its general hardness is 400ppm CaCO3. So its Residual Alkalinity may be estimated to be 250 - (400/4) = 150ppm.
If I were to use acid to drop the actual (measurable) alkalinity to 50ppm CaCO3 with acid, the general harness would remain the same and RA = -50ppm CaCO3.
So even though the measured alkalinity is 50ppm CaCO3, it is said the RA is minus 50ppm CaCO3.
Without patience, life becomes difficult and the sooner it's finished, the better.
-
SiHoltye
Re: Brun Water - RA & Bicarbonate goes minus, OK?
As well as all this is the -29 alkalinity figure ok?
Re: Brun Water - RA & Bicarbonate goes minus, OK?
Yes.
As stated above negative alkalinity isn't actually real alkalinity measure. It means there are is no bicarbonate left to "absorb" acid produced during the mash (which will lower the pH). As long as the predicted pH is where you want it you should be fine..
As stated above negative alkalinity isn't actually real alkalinity measure. It means there are is no bicarbonate left to "absorb" acid produced during the mash (which will lower the pH). As long as the predicted pH is where you want it you should be fine..

