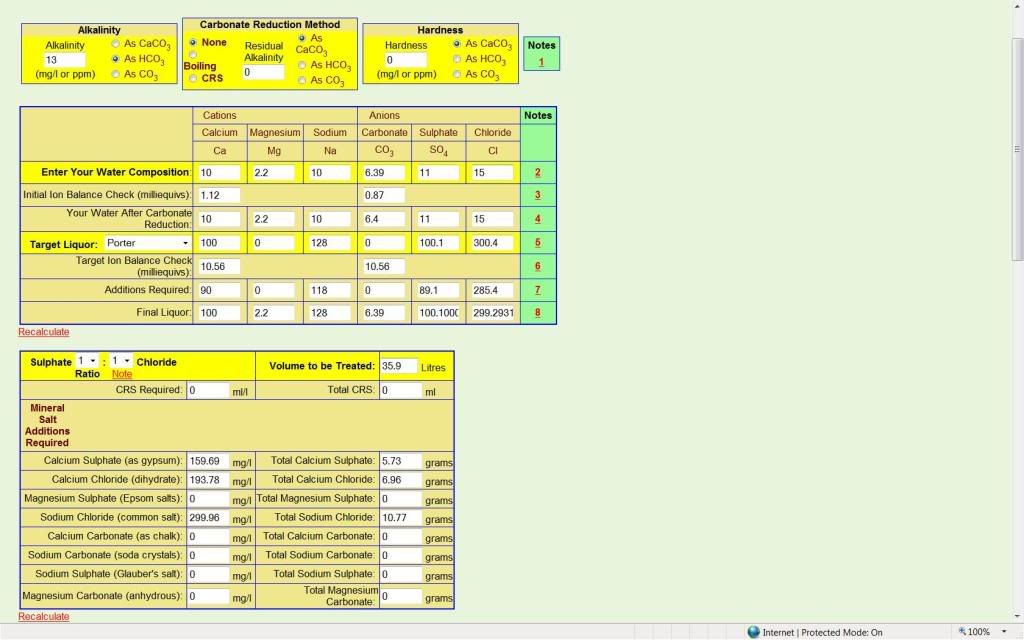
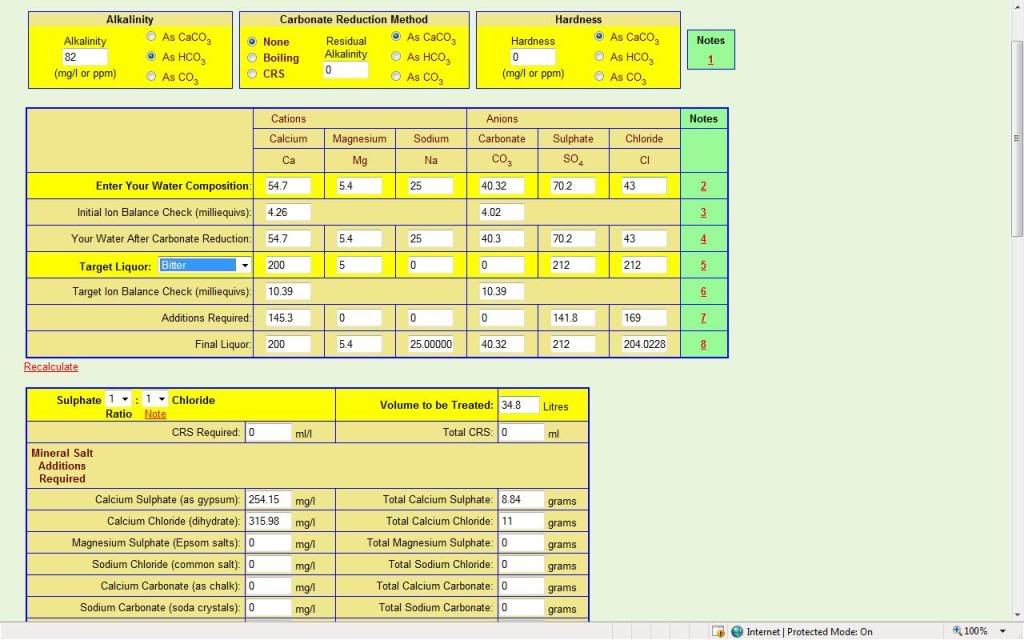
I'm lazy and have a habit of going off half-cocked and sounding silly, I'm doing it again now probably but.....
I half listened to an archived Basic Brewing Radio broadcast where ph was being discussed http://media.libsyn.com/media/basicbrew ... -11-07.mp3, both ph of the mash and of finished beer. Discussed was also what high/low readings might mean for you and the tastes you might encounter. Then I measured my finished beer to find what I think is a below good range ph reading.

Are my eyes OK? Does that strip look beneath ph3.8 to you too? When I have time I'll listen to the whole broadcast again and pay more attention...promise.