Well done, a lot of work there.
Best wishes for producing a beer mash pH predictor. I never intended going that far due to variation in malts by country and maltster and similar for adjuncts, never mind variations in crush and liquor to grist ratios and others I've not even considered. Frankly, by the time you've done a handful of brews of preferred recipes using your supply water, in your gear, by your methods, it's likely you can reasonably predict in your head what needs doing to get a suitable pH. Attempting to derive an algorithm converting your predictions for brewers using different equipment, other brewing liquor with estimated ion content for a single pot or BIAB or 3V and RIMS or HERMS is vastly more complicated.
My water varies substantially, and some years since several samples taken over a period that covered the full range of anticipated variation were analysed by Wallybrew. Samples of those waters were kept to recalibrated a TDS meter after analysis.
Each ion was plotted against Total Dissolved Solids as found in the analyses in Excel, passing through the origin of the graph as zero TDS means zero ion content. Excel's Chart Function will derive a mathematical function for plotted curves, and fed back into Excel enables determination of individual ion content from a single reading of the calibrated TDS meter in that water. This eliminates the initial GH and KH tests, but a KH test is taken after treatment and confirms all to be well. A Salifert kit now lasts me 5 years and more.
My water is from a single source, those analyses proved that and variation in mineral content found to be lower in periods of heavy rain and higher in drought. Your variation might be due to a change in the source and what I do might not help. If you name a major town near you, I might have data on historical mineral content to compare with your findings.
My system is mostly 3V, even when my single pot system is put to use. My water source usually contains similar quantity of sulphate, but all other major ions are higher, so after suitable alkalinity reduction, it is possible mash without adding salts without an excessively high pH. Salts are added to achieve a desired sulphate to chloride ratio as well as pH, which most commonly have a total weight of 10g of gypsum and/or Calcium Chloride Flake. By measuring mash pH after conversion is well underway, half and hour plus for British malts, some of the calcium salts can be added if necessary, usually the case, and the rest added to the boiler during the sparge.
With fly sparging, as the buffering properties of the sugars reduce by being transferred to the wort in the boiler, I will acid to the sparge liquor which starts at the same level as mash liquor, to stop pH rising above pH 5.6 or so.
This is a picture my Excel calculator. All inputs are entered in column B.
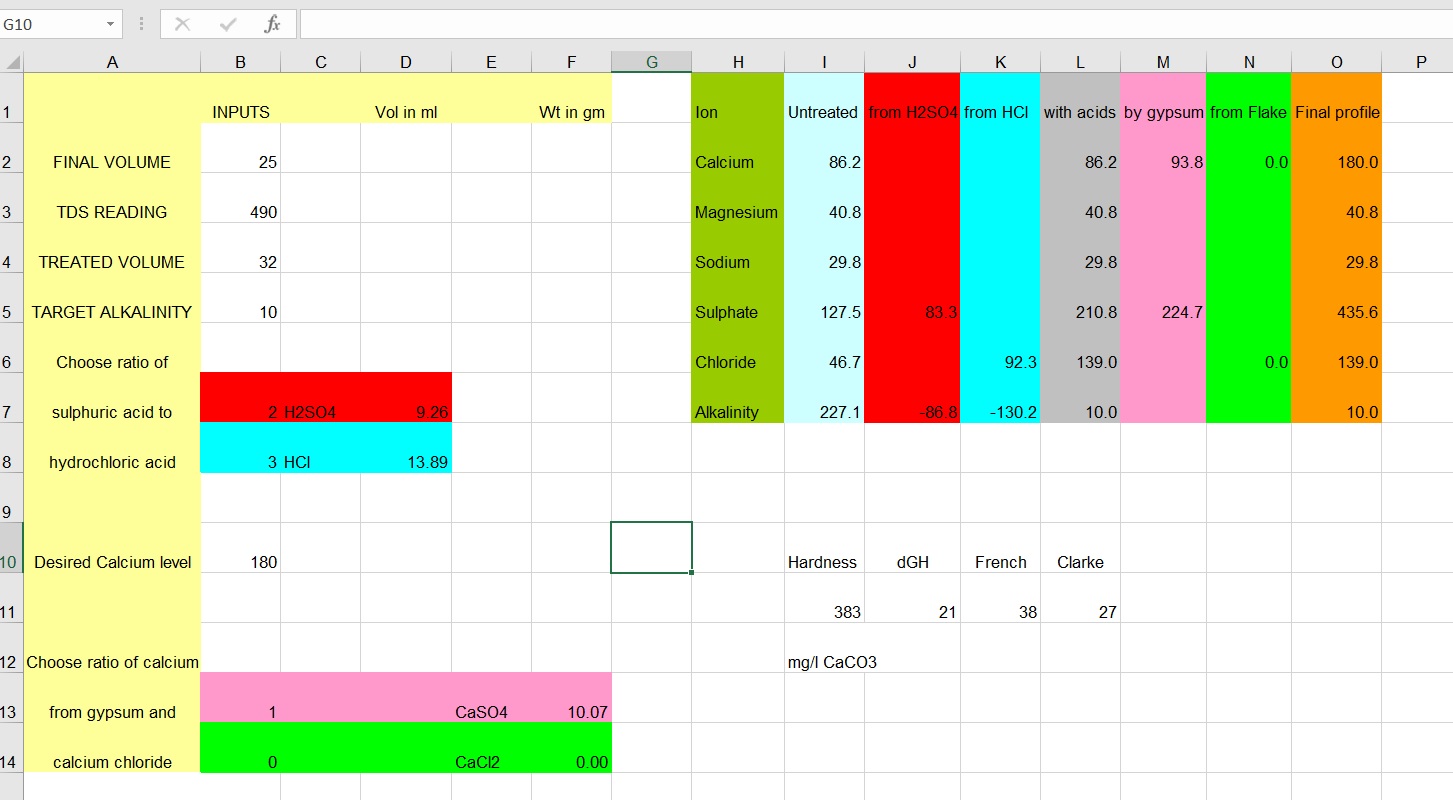
- BitterProfile.jpg (171.73 KiB) Viewed 302 times
Without patience, life becomes difficult and the sooner it's finished, the better.

