orlando wrote:
I don't understand why there appears to be a somewhat negative attitude to phosphoric. It is the most neutral acid as its acid is closest to the acids in the malt (phosphatic) does not introduce sulphate or chloride, which may not be desired so another adjustment needs to be made, is a little easier to handle as it doesn't produce vapours that are to be avoided and won't give you the trots like lactic if you use too much. Getting the rust off your car bodywork sounds like another plus to me

.
Hi Orlando,
Take Bigdave's water in this thread as an example. Why use phosphoric acid to reduce excess alkalinity when sulphuric acid or even CRS would both do the job and provide some of the calcium salts it lacks?
I'm far from convinced phosphoric acid is so called neutral and without flavour, is it a component in Coke for that reason? I've never knowingly found a defect that I would call minerality in my beer and to be frank, in any commercial one either. Try a Google with "minerality in wine" and "minerality in beer" to see how common such events are.
For some time I have thought that too much American theory on liquor for brewing is based upon the 1941 paper by Kohlbach. It apparently (I've read the paper was lost during the war) explained the anomaly of high mash pH with very low alkaline water was due to the equivalent lack of calcium salts in that water. He derived a formula to predicted pH change dependent upon variation of alkalinity and calcium salts. While all this was good stuff, I'm not convinced that the experience detailed in that paper is simply transferable to all, those made in a different way with different ingredients and different water.
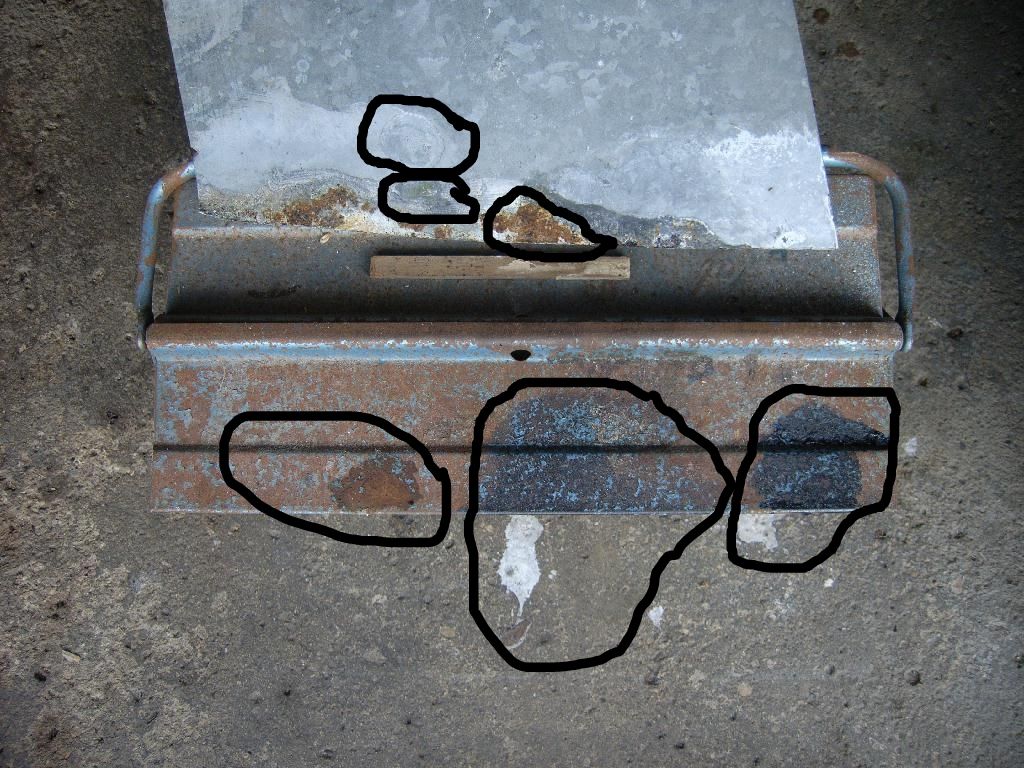
This was a test with sulphuric and phosphoric acid (don't ask why) on rusty steel and galvanised plate.
You can see how phoshoric removes rust (2 areas bottom right) but look at what happened to the concrete (mostly made of calcium compounds) where it spilled.
There was similar spillage with the sulphuric acid to the left but the result was soluable and washed away.
The marks from the phosphoric acid are still there more than a month later and they don't wash off.
I clean my kettle element with a soft cloth and finish with a toothbrush. Not sure how it would if with phosphoric acid.
Without patience, life becomes difficult and the sooner it's finished, the better.


