I've not used lactic acid for alkalinity reduction, but the consensus of opinion from those that have suggests that it can leave undesirable tastes in the beer. I've used phosphoric to good effect, but now I use sulphuric in order to manage sulphate levels as well as alkalinity.Fido97 wrote: 1. Rather than add CRS (expensive/mystery ingredients) could I add some other acid such as Lactic Acid or Phosphoric Acid? If so what amounts would be required to reduce alkalinity. I see there is a very good explanation of how much CRS is needed but is there a similar guide for adding alternative acids?
Water chemistry for idiots
-
Dave S
- Even further under the Table
- Posts: 2514
- Joined: Sun Apr 01, 2012 5:38 pm
- Location: Wirral, Merseyside
Re: Water chemistry for idiots
Best wishes
Dave
Dave
-
Fido97
Re: Water chemistry for idiots
Thanks Dave S. Where do you get your sulphuric acid and is there a conversion table/formula to reduce alkalinity? Also in what form is the acid - liquid - concentrated/diluted/dry? Cheers,
-
Dave S
- Even further under the Table
- Posts: 2514
- Joined: Sun Apr 01, 2012 5:38 pm
- Location: Wirral, Merseyside
Re: Water chemistry for idiots
Well now you're potentially opening up a can of worms. I, along with a lot of people here get my sulphuric from Murphy. There have been issues with them though over the the units they are using for concentration claims. My bottle states 25% W/W, which it is not. It has been discovered by people more knowledgeable in chemistry here that it is in fact 25% v/v. But don't take that for granted, it seems to be a little variableFido97 wrote:Thanks Dave S. Where do you get your sulphuric acid and is there a conversion table/formula to reduce alkalinity? Also in what form is the acid - liquid - concentrated/diluted/dry? Cheers,
Regarding conversion tables, There are a number water calculators that will tell you how much to use, depending on the strength and target alkalinity. There is Graham's calculator, accessible from the tabs at the top of these pages or there is Bru 'n Water. The latter has a free version and a supporters' version, which costs whatever you wish to pay. It was written by Martin Brungard who is a member here. If you PM him I'm sure he will help.
EDIT: You may wish to read this thread for more background.
Best wishes
Dave
Dave
- barneey
- Telling imaginary friend stories
- Posts: 5423
- Joined: Mon Jul 25, 2011 10:42 pm
- Location: East Kent
Re: Water chemistry for idiots
Whatever acid you decide to buy from whatever source, it is recommended that you confirm the strength of the Acid first to make sure it is what is says it is on the bottle.
To carry out the test you will need to be able to measure Alkalinity, 1st of the untreated example and second of the treated sample.
Make sure you measure both the Alkalinity and Acid used as accurately as you can. (MIX VERY WELL)
Post on JBK the Alkalinity of the water before treatment and the Alkalinity of the Water after Acid treatment & the amount of Acid used, we (me) can then confirm what the concentration of the Acid is.
As with most forms of water treatment, I would always recommend that you test the alkalinity before and after treatment, DONT just chuck a required amount of acid into the water and except what the calculator is telling you is the truth.
To carry out the test you will need to be able to measure Alkalinity, 1st of the untreated example and second of the treated sample.
Make sure you measure both the Alkalinity and Acid used as accurately as you can. (MIX VERY WELL)
Post on JBK the Alkalinity of the water before treatment and the Alkalinity of the Water after Acid treatment & the amount of Acid used, we (me) can then confirm what the concentration of the Acid is.
As with most forms of water treatment, I would always recommend that you test the alkalinity before and after treatment, DONT just chuck a required amount of acid into the water and except what the calculator is telling you is the truth.
Hair of the dog, bacon, butty.
Hops, cider pips & hello.
Name the Movie + song :)
Hops, cider pips & hello.
Name the Movie + song :)
- Monkeybrew
- Telling everyone Your My Best Mate
- Posts: 4104
- Joined: Wed Apr 13, 2011 9:53 pm
- Location: Essex
Re: Water chemistry for idiots
Hi
I have been reading a few threads in this Brewing Liquor forum, and was drawn to the title of this topic as I thought that it probably applied to me. I have used CRS & DLS in my last 4 AG brews, but have calculated my additions based on my Anglian Water report that actually states the Alkalinity (296) and Calcium (118).
I realise after reading Aleman's great articles at the top of this site and other posts here, that this isn't an accurate method.
Is this the correct Salifert that I need?
http://www.fish-fish-fish.com/salifert- ... tAodul8A_A
Cheers and sorry for the hijack.
MB
I have been reading a few threads in this Brewing Liquor forum, and was drawn to the title of this topic as I thought that it probably applied to me. I have used CRS & DLS in my last 4 AG brews, but have calculated my additions based on my Anglian Water report that actually states the Alkalinity (296) and Calcium (118).
I realise after reading Aleman's great articles at the top of this site and other posts here, that this isn't an accurate method.
Is this the correct Salifert that I need?
http://www.fish-fish-fish.com/salifert- ... tAodul8A_A
Cheers and sorry for the hijack.
MB
FV:
Conditioning:
AG#41 - Vienna Lager - 5.6%
AG#42 - Heritage Double Ale - 10.5%
On Tap:
AG#44 - Harvest ESB - 5.4%
AG#45 - Amarillo Gold APA - 5.2%
Conditioning:
AG#41 - Vienna Lager - 5.6%
AG#42 - Heritage Double Ale - 10.5%
On Tap:
AG#44 - Harvest ESB - 5.4%
AG#45 - Amarillo Gold APA - 5.2%
-
Dave S
- Even further under the Table
- Posts: 2514
- Joined: Sun Apr 01, 2012 5:38 pm
- Location: Wirral, Merseyside
Re: Water chemistry for idiots
That's the stuff, and a good price too. BTW, I don't find your post a hijack. It seems to fit perfectly well with the thread to me.Monkeybrew wrote:Hi
I have been reading a few threads in this Brewing Liquor forum, and was drawn to the title of this topic as I thought that it probably applied to me. I have used CRS & DLS in my last 4 AG brews, but have calculated my additions based on my Anglian Water report that actually states the Alkalinity (296) and Calcium (118).
I realise after reading Aleman's great articles at the top of this site and other posts here, that this isn't an accurate method.
Is this the correct Salifert that I need?
http://www.fish-fish-fish.com/salifert- ... tAodul8A_A
Cheers and sorry for the hijack.
MB
Best wishes
Dave
Dave
- Monkeybrew
- Telling everyone Your My Best Mate
- Posts: 4104
- Joined: Wed Apr 13, 2011 9:53 pm
- Location: Essex
Re: Water chemistry for idiots
Cheers Dave, all ordered 
MB
MB
FV:
Conditioning:
AG#41 - Vienna Lager - 5.6%
AG#42 - Heritage Double Ale - 10.5%
On Tap:
AG#44 - Harvest ESB - 5.4%
AG#45 - Amarillo Gold APA - 5.2%
Conditioning:
AG#41 - Vienna Lager - 5.6%
AG#42 - Heritage Double Ale - 10.5%
On Tap:
AG#44 - Harvest ESB - 5.4%
AG#45 - Amarillo Gold APA - 5.2%
Water chemistry for idiots
Yep
Edit: doh, somehow missed the reply!
Edit: doh, somehow missed the reply!
- Monkeybrew
- Telling everyone Your My Best Mate
- Posts: 4104
- Joined: Wed Apr 13, 2011 9:53 pm
- Location: Essex
Re: Water chemistry for idiots
Monkeybrew wrote:Hi
I have been reading a few threads in this Brewing Liquor forum, and was drawn to the title of this topic as I thought that it probably applied to me. I have used CRS & DLS in my last 4 AG brews, but have calculated my additions based on my Anglian Water report that actually states the Alkalinity (296) and Calcium (118).
I realise after reading Aleman's great articles at the top of this site and other posts here, that this isn't an accurate method.
Is this the correct Salifert that I need?
http://www.fish-fish-fish.com/salifert- ... tAodul8A_A
Cheers and sorry for the hijack.
MB
Well, my Salifert kit arrived this afternoon, so now that the kids are in bed and some calm has been restored, I have tested my water.
Bearing in mind that Anglian water tested my supply as Alkalinity CaC03 296ppm & Calcium 118ppm, the results from the Salifert test kit were actually CaC03 179.5ppm! That's a massive difference
The Salifert kit is certainly nice and easy to use, and I ended up with 0.35ml of the KH reagent in the 1ml syinge as the solution flashed to pink and then with one more drop it stayed pink. Just checking that I did this correct, that meant that my Alkalinity in meq/L was 3.59 and I multiplied this by 50, correct?
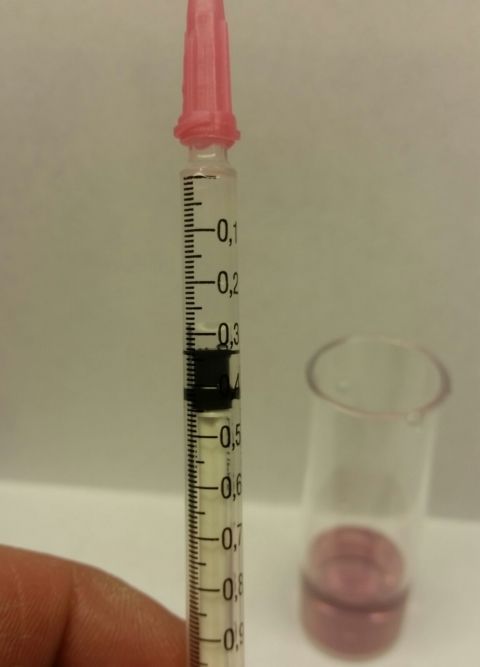
Cheers
MB
Edit - Can I assume that my Calcium is 179.5 x 0.4?
FV:
Conditioning:
AG#41 - Vienna Lager - 5.6%
AG#42 - Heritage Double Ale - 10.5%
On Tap:
AG#44 - Harvest ESB - 5.4%
AG#45 - Amarillo Gold APA - 5.2%
Conditioning:
AG#41 - Vienna Lager - 5.6%
AG#42 - Heritage Double Ale - 10.5%
On Tap:
AG#44 - Harvest ESB - 5.4%
AG#45 - Amarillo Gold APA - 5.2%
- GrowlingDogBeer
- Even further under the Table
- Posts: 2671
- Joined: Tue Aug 17, 2010 5:20 pm
- Location: Wickford, Essex
- Contact:
Re: Water chemistry for idiots
Looks like you got your Alkalinity correct.
I'm not sure that calculation to work out the Calcium is correct though, I'm sure one of the experts will soon be along to help.
I'm not sure that calculation to work out the Calcium is correct though, I'm sure one of the experts will soon be along to help.
- Eric
- Even further under the Table
- Posts: 2918
- Joined: Fri Mar 13, 2009 1:18 am
- Location: Sunderland.
Re: Water chemistry for idiots
Calcium makes up 40% of the weight of calcium carbonate.
What you have measured is all alkalinity in that sample of water measured as if it was entirely due to the presence of calcium carbonate. However, not all that alkalinity will solely be due from calcium, it is highly probable that some is attached to magnesium, then on the other hand there will be some calcium present not providing alkalinity.
What you do have is a better idea of how much calcium is likely present and the ability to accurately treat alkalinity to match the grain in a mash.
What you have measured is all alkalinity in that sample of water measured as if it was entirely due to the presence of calcium carbonate. However, not all that alkalinity will solely be due from calcium, it is highly probable that some is attached to magnesium, then on the other hand there will be some calcium present not providing alkalinity.
What you do have is a better idea of how much calcium is likely present and the ability to accurately treat alkalinity to match the grain in a mash.
Without patience, life becomes difficult and the sooner it's finished, the better.

