Reducing alkalinity using acid.
-
Dave S
- Even further under the Table
- Posts: 2514
- Joined: Sun Apr 01, 2012 5:38 pm
- Location: Wirral, Merseyside
Re: Reducing alkalinity using acid.
Good point. If they're saying it's food grade though, it won't be mixed with anything nasty.
Best wishes
Dave
Dave
- orlando
- So far gone I'm on the way back again!
- Posts: 7201
- Joined: Thu Nov 17, 2011 3:22 pm
- Location: North Norfolk: Nearest breweries All Day Brewery, Salle. Panther, Reepham. Yetman's, Holt
Re: Reducing alkalinity using acid.
What I didn't mention was I changed the % in my calculator to work out the average so the figures above take this into account.Dave S wrote:orlando wrote:Right Dave, it's taken me a couple of days but I now have a supplier of food grade phosphoric acid at 85% strength. I have ordered 2 x 500 ml as strangely this was cheaper than 1 litre. I ordered 2 because the courier charge is the same for one or two and as that is £8.75, so it made more sense. You have to register with the site because of what is going through the post and I suppose that accounts for the hefty charge. They work out at £6.41 each inc. vat so with courier that comes to £21.57. I reckon that using approx. 20ml per brew you are looking at 25 brews per bottle, so just over 40p per brew.
You can get it from here. They take PayPal too.
Great, thanks Orlando. That looks like a very useful site. Plus, being 85% means that you'd use less per brew anyway compared with the 75% one.
I am "The Little Red Brooster"
Fermenting:
Conditioning:
Drinking: Southwold Again,
Up Next: John Barleycorn (Barley Wine)
Planning: Winter drinking Beer
Fermenting:
Conditioning:
Drinking: Southwold Again,
Up Next: John Barleycorn (Barley Wine)
Planning: Winter drinking Beer
- orlando
- So far gone I'm on the way back again!
- Posts: 7201
- Joined: Thu Nov 17, 2011 3:22 pm
- Location: North Norfolk: Nearest breweries All Day Brewery, Salle. Panther, Reepham. Yetman's, Holt
Re: Reducing alkalinity using acid.
No but I have been assured that it is food grade and I have asked for the MSDS as confirmation. Will confirm all this when I get it.barneey wrote:orlando wrote:Right Dave, it's taken me a couple of days but I now have a supplier of food grade phosphoric acid at 85% strength. I have ordered 2 x 500 ml as strangely this was cheaper than 1 litre. I ordered 2 because the courier charge is the same for one or two and as that is £8.75, so it made more sense. You have to register with the site because of what is going through the post and I suppose that accounts for the hefty charge. They work out at £6.41 each inc. vat so with courier that comes to £21.57. I reckon that using approx. 20ml per brew you are looking at 25 brews per bottle, so just over 40p per brew.
You can get it from here. They take PayPal too.
Did they tell you the exavt make up of the acid?
Cheers
I am "The Little Red Brooster"
Fermenting:
Conditioning:
Drinking: Southwold Again,
Up Next: John Barleycorn (Barley Wine)
Planning: Winter drinking Beer
Fermenting:
Conditioning:
Drinking: Southwold Again,
Up Next: John Barleycorn (Barley Wine)
Planning: Winter drinking Beer
- orlando
- So far gone I'm on the way back again!
- Posts: 7201
- Joined: Thu Nov 17, 2011 3:22 pm
- Location: North Norfolk: Nearest breweries All Day Brewery, Salle. Panther, Reepham. Yetman's, Holt
Re: Reducing alkalinity using acid.
I received the MSDS in PDF format and have copied it below. Apologies for the formatting as I don't know how to post this any other way, lesson welcomed.
A quick (very) skim through hasn't pinpointed specific reference to my untrained eye as to its suitability for food use so have emailed for further confirmation, again will post accordingly.
SAFETY DATA SHEET
PHOSPHORIC ACID 85%
Page: 1
Compilation date: 27/02/2012
Revision No: 1
Section 1: Identification of the substance/mixture and of the company/undertaking
1.1. Product identifier
Product name: PHOSPHORIC ACID 85%
CAS number: 7664-38-2
Product code: GPC8046
1.2. Relevant identified uses of the substance or mixture and uses advised against
1.3. Details of the supplier of the safety data sheet
Company name: Atom Scientific Ltd
Unit 6A
Arrow Trading Estate
Audenshaw
Manchester
M34 5LR
United Kingdom
Tel: 0161 320 0022
Fax: 01704 337 167
Email: enquiries@atomscientific.com
1.4. Emergency telephone number
Emergency tel: 0161 320 0022
(office hours only)
Section 2: Hazards identification
2.1. Classification of the substance or mixture
Classification under CHIP: C: R34
Classification under CLP: Skin Corr. 1B: H314
Most important adverse effects: Causes burns.
2.2. Label elements
Label elements under CLP:
Hazard statements: H314: Causes severe skin burns and eye damage.
Signal words: Danger
Hazard pictograms: GHS05: Corrosion
Precautionary statements: P264: Wash hands thoroughly after handling.
[cont...]
SAFETY DATA SHEET
PHOSPHORIC ACID 85%
Page: 2
P280: Wear protective gloves/protective clothing/eye protection/face protection.
P305+351+338: IF IN EYES: Rinse cautiously with water for several minutes. Remove
contact lenses, if present and easy to do. Continue rinsing.
P321: Specific treatment (see advice on this label).
P405: Store locked up.
P501: Dispose of contents/container to an approved waste disposal company.
P310: Immediately call a POISON CENTER or doctor.
Label elements under CHIP:
Hazard symbols: Corrosive.
Risk phrases: R34: Causes burns.
Safety phrases: S26: In case of contact with eyes, rinse immediately with plenty of water and seek
medical advice.
S36/37/39: Wear suitable protective clothing, gloves and eye / face protection.
S45: In case of accident or if you feel unwell, seek medical advice immediately (show
the label where possible).
2.3. Other hazards
PBT: This substance is not identified as a PBT substance.
Section 3: Composition/information on ingredients
3.2. Mixtures
Hazardous ingredients:
ORTHOPHOSPHORIC ACID
EINECS CAS CHIP Classification CLP Classification Percent
231-633-2 7664-38-2 C: R34 Skin Corr. 1B: H314 70-90%
Section 4: First aid measures
4.1. Description of first aid measures
Skin contact: Remove all contaminated clothes and footwear immediately unless stuck to skin.
Drench the affected skin with running water for 10 minutes or longer if substance is still
on skin. Transfer to hospital if there are burns or symptoms of poisoning.
Eye contact: Bathe the eye with running water for 15 minutes. Transfer to hospital for specialist
examination.
Ingestion: Wash out mouth with water. Do not induce vomiting. Transfer to hospital as soon as
possible.
Inhalation: Move to fresh air in case of accidental inhalation of vapours. If unconscious, check for
breathing and apply artificial respiration if necessary. Consult a doctor.
[cont...]
SAFETY DATA SHEET
PHOSPHORIC ACID 85%
Page: 3
4.2. Most important symptoms and effects, both acute and delayed
Skin contact: May be harmful if absorbed through the skin Severe burns may occur.
Eye contact: Corneal burns may occur. May cause permanent damage.
Ingestion: May be harmful if swallowed Causes burns Corrosive burns may appear around the
lips.
Inhalation: There may be shortness of breath with a burning sensation in the throat. Exposure may
cause coughing or wheezing. The substance is destructive to the respiratory tract and
mucous membranes.
Delayed / immediate effects: Immediate effects can be expected after short-term exposure.
4.3. Indication of any immediate medical attention and special treatment needed
Immediate / special treatment: Eye bathing equipment should be available on the premises.
Section 5: Fire-fighting measures
5.1. Extinguishing media
Extinguishing media: Suitable extinguishing media for the surrounding fire should be used. Use water spray
to cool containers.
5.2. Special hazards arising from the substance or mixture
Exposure hazards: Corrosive. In combustion emits toxic fumes of phosphorous oxides.
5.3. Advice for fire-fighters
Advice for fire-fighters: Wear self-contained breathing apparatus. Wear protective clothing to prevent contact
with skin and eyes.
Section 6: Accidental release measures
6.1. Personal precautions, protective equipment and emergency procedures
Personal precautions: Refer to section 8 of SDS for personal protection details. Avoid breathing vapours, mist
or gas Avoid breathing dust. Evacuate the area immediately.
6.2. Environmental precautions
Environmental precautions: Do not discharge into drains or rivers. Contain the spillage using bunding.
6.3. Methods and material for containment and cleaning up
Clean-up procedures: Absorb into dry earth or sand. Transfer to a closable, labelled salvage container for
disposal by an appropriate method.
6.4. Reference to other sections
Reference to other sections: Refer to section 8 of SDS.
Section 7: Handling and storage
[cont...]
SAFETY DATA SHEET
PHOSPHORIC ACID 85%
Page: 4
7.1. Precautions for safe handling
Handling requirements: Avoid direct contact with the substance. Ensure there is sufficient ventilation of the area.
Avoid the formation or spread of mists in the air.
7.2. Conditions for safe storage, including any incompatibilities
Storage conditions: Store in cool, well ventilated area. Keep container tightly closed.
7.3. Specific end use(s)
Specific end use(s): No data available.
Section 8: Exposure controls/personal protection
8.1. Control parameters
Hazardous ingredients:
ORTHOPHOSPHORIC ACID...100%
Workplace exposure limits: Respirable dust
State 8 hour TWA 15 min. STEL 8 hour TWA 15 min. STEL
UK 1 mg/m3 2 mg/m3 - -
8.2. Exposure controls
Engineering measures: Ensure there is sufficient ventilation of the area.
Respiratory protection: Self-contained breathing apparatus must be available in case of emergency.
Hand protection: Impermeable gloves.
Eye protection: Tightly fitting safety goggles. Ensure eye bath is to hand.
Skin protection: Impermeable protective clothing.
Section 9: Physical and chemical properties
9.1. Information on basic physical and chemical properties
State: Liquid
Colour: Colourless
Boiling point/range°C: 158 Melting point/range°C: 40
Relative density: 1.685 g/ml at 25C
9.2. Other information
Other information: Not applicable.
Section 10: Stability and reactivity
10.1. Reactivity
Reactivity: Stable under recommended transport or storage conditions.
[cont...]
SAFETY DATA SHEET
PHOSPHORIC ACID 85%
Page: 5
10.2. Chemical stability
Chemical stability: Stable under normal conditions.
10.3. Possibility of hazardous reactions
Hazardous reactions: Hazardous reactions will not occur under normal transport or storage conditions.
Decomposition may occur on exposure to conditions or materials listed below.
10.4. Conditions to avoid
Conditions to avoid: Heat.
10.5. Incompatible materials
Materials to avoid: Strong bases. Powdered Metals
10.6. Hazardous decomposition products
Haz. decomp. products: In combustion emits toxic fumes.
Section 11: Toxicological information
11.1. Information on toxicological effects
Relevant effects for mixture:
Effect Route Basis
Corrosivity OPT INH DRM Hazardous: calculated
Symptoms / routes of exposure
Skin contact: May be harmful if absorbed through the skin Severe burns may occur.
Eye contact: Corneal burns may occur. May cause permanent damage.
Ingestion: May be harmful if swallowed Causes burns Corrosive burns may appear around the
lips.
Inhalation: There may be shortness of breath with a burning sensation in the throat. Exposure may
cause coughing or wheezing. The substance is destructive to the respiratory tract and
mucous membranes.
Delayed / immediate effects: Immediate effects can be expected after short-term exposure.
Section 12: Ecological information
12.1. Toxicity
Ecotoxicity values: Not applicable.
12.2. Persistence and degradability
12.3. Bioaccumulative potential
12.4. Mobility in soil
[cont...]
SAFETY DATA SHEET
PHOSPHORIC ACID 85%
Page: 6
12.5. Results of PBT and vPvB assessment
PBT identification: This substance is not identified as a PBT substance.
12.6. Other adverse effects
Section 13: Disposal considerations
13.1. Waste treatment methods
Disposal operations: Transfer to a suitable container and arrange for collection by specialised disposal
company.
Disposal of packaging: Arrange for collection by specialised disposal company.
NB: The user's attention is drawn to the possible existence of regional or national
regulations regarding disposal.
Section 14: Transport information
14.1. UN number
UN number: UN1805
14.2. UN proper shipping name
Shipping name: PHOSPHORIC ACID, SOLUTION
14.3. Transport hazard class(es)
Transport class: 8
14.4. Packing group
Packing group: III
14.5. Environmental hazards
Environmentally hazardous: No Marine pollutant: No
14.6. Special precautions for user
Special precautions: No special precautions.
Tunnel code: E
Transport category: 3
Section 15: Regulatory information
15.1. Safety, health and environmental regulations/legislation specific for the substance or mixture
Specific regulations: This safety datasheet complies with the requirements of Regulation (EC) No.
1907/2006.
15.2. Chemical Safety Assessment
Chemical safety assessment: A chemical safety assessment has not been carried out for the substance or the mixture
by the supplier.
[cont...]
SAFETY DATA SHEET
PHOSPHORIC ACID 85%
Page: 7
Section 16: Other information
Other information
Other information: This safety data sheet is prepared in accordance with Commission Regulation (EU) No
453/2010.
* indicates text in the SDS which has changed since the last revision.
Phrases used in s.2 and 3: H314: Causes severe skin burns and eye damage.
R34: Causes burns.
Legal disclaimer: The above information is believed to be correct but does not purport to be all inclusive
and shall be used only as a guide. This company shall not be held liable for any
damage resulting from handling or from contact with the above product.
[final page]
A quick (very) skim through hasn't pinpointed specific reference to my untrained eye as to its suitability for food use so have emailed for further confirmation, again will post accordingly.
SAFETY DATA SHEET
PHOSPHORIC ACID 85%
Page: 1
Compilation date: 27/02/2012
Revision No: 1
Section 1: Identification of the substance/mixture and of the company/undertaking
1.1. Product identifier
Product name: PHOSPHORIC ACID 85%
CAS number: 7664-38-2
Product code: GPC8046
1.2. Relevant identified uses of the substance or mixture and uses advised against
1.3. Details of the supplier of the safety data sheet
Company name: Atom Scientific Ltd
Unit 6A
Arrow Trading Estate
Audenshaw
Manchester
M34 5LR
United Kingdom
Tel: 0161 320 0022
Fax: 01704 337 167
Email: enquiries@atomscientific.com
1.4. Emergency telephone number
Emergency tel: 0161 320 0022
(office hours only)
Section 2: Hazards identification
2.1. Classification of the substance or mixture
Classification under CHIP: C: R34
Classification under CLP: Skin Corr. 1B: H314
Most important adverse effects: Causes burns.
2.2. Label elements
Label elements under CLP:
Hazard statements: H314: Causes severe skin burns and eye damage.
Signal words: Danger
Hazard pictograms: GHS05: Corrosion
Precautionary statements: P264: Wash hands thoroughly after handling.
[cont...]
SAFETY DATA SHEET
PHOSPHORIC ACID 85%
Page: 2
P280: Wear protective gloves/protective clothing/eye protection/face protection.
P305+351+338: IF IN EYES: Rinse cautiously with water for several minutes. Remove
contact lenses, if present and easy to do. Continue rinsing.
P321: Specific treatment (see advice on this label).
P405: Store locked up.
P501: Dispose of contents/container to an approved waste disposal company.
P310: Immediately call a POISON CENTER or doctor.
Label elements under CHIP:
Hazard symbols: Corrosive.
Risk phrases: R34: Causes burns.
Safety phrases: S26: In case of contact with eyes, rinse immediately with plenty of water and seek
medical advice.
S36/37/39: Wear suitable protective clothing, gloves and eye / face protection.
S45: In case of accident or if you feel unwell, seek medical advice immediately (show
the label where possible).
2.3. Other hazards
PBT: This substance is not identified as a PBT substance.
Section 3: Composition/information on ingredients
3.2. Mixtures
Hazardous ingredients:
ORTHOPHOSPHORIC ACID
EINECS CAS CHIP Classification CLP Classification Percent
231-633-2 7664-38-2 C: R34 Skin Corr. 1B: H314 70-90%
Section 4: First aid measures
4.1. Description of first aid measures
Skin contact: Remove all contaminated clothes and footwear immediately unless stuck to skin.
Drench the affected skin with running water for 10 minutes or longer if substance is still
on skin. Transfer to hospital if there are burns or symptoms of poisoning.
Eye contact: Bathe the eye with running water for 15 minutes. Transfer to hospital for specialist
examination.
Ingestion: Wash out mouth with water. Do not induce vomiting. Transfer to hospital as soon as
possible.
Inhalation: Move to fresh air in case of accidental inhalation of vapours. If unconscious, check for
breathing and apply artificial respiration if necessary. Consult a doctor.
[cont...]
SAFETY DATA SHEET
PHOSPHORIC ACID 85%
Page: 3
4.2. Most important symptoms and effects, both acute and delayed
Skin contact: May be harmful if absorbed through the skin Severe burns may occur.
Eye contact: Corneal burns may occur. May cause permanent damage.
Ingestion: May be harmful if swallowed Causes burns Corrosive burns may appear around the
lips.
Inhalation: There may be shortness of breath with a burning sensation in the throat. Exposure may
cause coughing or wheezing. The substance is destructive to the respiratory tract and
mucous membranes.
Delayed / immediate effects: Immediate effects can be expected after short-term exposure.
4.3. Indication of any immediate medical attention and special treatment needed
Immediate / special treatment: Eye bathing equipment should be available on the premises.
Section 5: Fire-fighting measures
5.1. Extinguishing media
Extinguishing media: Suitable extinguishing media for the surrounding fire should be used. Use water spray
to cool containers.
5.2. Special hazards arising from the substance or mixture
Exposure hazards: Corrosive. In combustion emits toxic fumes of phosphorous oxides.
5.3. Advice for fire-fighters
Advice for fire-fighters: Wear self-contained breathing apparatus. Wear protective clothing to prevent contact
with skin and eyes.
Section 6: Accidental release measures
6.1. Personal precautions, protective equipment and emergency procedures
Personal precautions: Refer to section 8 of SDS for personal protection details. Avoid breathing vapours, mist
or gas Avoid breathing dust. Evacuate the area immediately.
6.2. Environmental precautions
Environmental precautions: Do not discharge into drains or rivers. Contain the spillage using bunding.
6.3. Methods and material for containment and cleaning up
Clean-up procedures: Absorb into dry earth or sand. Transfer to a closable, labelled salvage container for
disposal by an appropriate method.
6.4. Reference to other sections
Reference to other sections: Refer to section 8 of SDS.
Section 7: Handling and storage
[cont...]
SAFETY DATA SHEET
PHOSPHORIC ACID 85%
Page: 4
7.1. Precautions for safe handling
Handling requirements: Avoid direct contact with the substance. Ensure there is sufficient ventilation of the area.
Avoid the formation or spread of mists in the air.
7.2. Conditions for safe storage, including any incompatibilities
Storage conditions: Store in cool, well ventilated area. Keep container tightly closed.
7.3. Specific end use(s)
Specific end use(s): No data available.
Section 8: Exposure controls/personal protection
8.1. Control parameters
Hazardous ingredients:
ORTHOPHOSPHORIC ACID...100%
Workplace exposure limits: Respirable dust
State 8 hour TWA 15 min. STEL 8 hour TWA 15 min. STEL
UK 1 mg/m3 2 mg/m3 - -
8.2. Exposure controls
Engineering measures: Ensure there is sufficient ventilation of the area.
Respiratory protection: Self-contained breathing apparatus must be available in case of emergency.
Hand protection: Impermeable gloves.
Eye protection: Tightly fitting safety goggles. Ensure eye bath is to hand.
Skin protection: Impermeable protective clothing.
Section 9: Physical and chemical properties
9.1. Information on basic physical and chemical properties
State: Liquid
Colour: Colourless
Boiling point/range°C: 158 Melting point/range°C: 40
Relative density: 1.685 g/ml at 25C
9.2. Other information
Other information: Not applicable.
Section 10: Stability and reactivity
10.1. Reactivity
Reactivity: Stable under recommended transport or storage conditions.
[cont...]
SAFETY DATA SHEET
PHOSPHORIC ACID 85%
Page: 5
10.2. Chemical stability
Chemical stability: Stable under normal conditions.
10.3. Possibility of hazardous reactions
Hazardous reactions: Hazardous reactions will not occur under normal transport or storage conditions.
Decomposition may occur on exposure to conditions or materials listed below.
10.4. Conditions to avoid
Conditions to avoid: Heat.
10.5. Incompatible materials
Materials to avoid: Strong bases. Powdered Metals
10.6. Hazardous decomposition products
Haz. decomp. products: In combustion emits toxic fumes.
Section 11: Toxicological information
11.1. Information on toxicological effects
Relevant effects for mixture:
Effect Route Basis
Corrosivity OPT INH DRM Hazardous: calculated
Symptoms / routes of exposure
Skin contact: May be harmful if absorbed through the skin Severe burns may occur.
Eye contact: Corneal burns may occur. May cause permanent damage.
Ingestion: May be harmful if swallowed Causes burns Corrosive burns may appear around the
lips.
Inhalation: There may be shortness of breath with a burning sensation in the throat. Exposure may
cause coughing or wheezing. The substance is destructive to the respiratory tract and
mucous membranes.
Delayed / immediate effects: Immediate effects can be expected after short-term exposure.
Section 12: Ecological information
12.1. Toxicity
Ecotoxicity values: Not applicable.
12.2. Persistence and degradability
12.3. Bioaccumulative potential
12.4. Mobility in soil
[cont...]
SAFETY DATA SHEET
PHOSPHORIC ACID 85%
Page: 6
12.5. Results of PBT and vPvB assessment
PBT identification: This substance is not identified as a PBT substance.
12.6. Other adverse effects
Section 13: Disposal considerations
13.1. Waste treatment methods
Disposal operations: Transfer to a suitable container and arrange for collection by specialised disposal
company.
Disposal of packaging: Arrange for collection by specialised disposal company.
NB: The user's attention is drawn to the possible existence of regional or national
regulations regarding disposal.
Section 14: Transport information
14.1. UN number
UN number: UN1805
14.2. UN proper shipping name
Shipping name: PHOSPHORIC ACID, SOLUTION
14.3. Transport hazard class(es)
Transport class: 8
14.4. Packing group
Packing group: III
14.5. Environmental hazards
Environmentally hazardous: No Marine pollutant: No
14.6. Special precautions for user
Special precautions: No special precautions.
Tunnel code: E
Transport category: 3
Section 15: Regulatory information
15.1. Safety, health and environmental regulations/legislation specific for the substance or mixture
Specific regulations: This safety datasheet complies with the requirements of Regulation (EC) No.
1907/2006.
15.2. Chemical Safety Assessment
Chemical safety assessment: A chemical safety assessment has not been carried out for the substance or the mixture
by the supplier.
[cont...]
SAFETY DATA SHEET
PHOSPHORIC ACID 85%
Page: 7
Section 16: Other information
Other information
Other information: This safety data sheet is prepared in accordance with Commission Regulation (EU) No
453/2010.
* indicates text in the SDS which has changed since the last revision.
Phrases used in s.2 and 3: H314: Causes severe skin burns and eye damage.
R34: Causes burns.
Legal disclaimer: The above information is believed to be correct but does not purport to be all inclusive
and shall be used only as a guide. This company shall not be held liable for any
damage resulting from handling or from contact with the above product.
[final page]
I am "The Little Red Brooster"
Fermenting:
Conditioning:
Drinking: Southwold Again,
Up Next: John Barleycorn (Barley Wine)
Planning: Winter drinking Beer
Fermenting:
Conditioning:
Drinking: Southwold Again,
Up Next: John Barleycorn (Barley Wine)
Planning: Winter drinking Beer
-
Dave S
- Even further under the Table
- Posts: 2514
- Joined: Sun Apr 01, 2012 5:38 pm
- Location: Wirral, Merseyside
Re: Reducing alkalinity using acid.
It's good that it's not PBT, (Persistent, Bioaccumulative and Toxic). This is good indication, I would say that it is food grade, though more information in that way would be good.orlando wrote:I received the MSDS in PDF format and have copied it below. Apologies for the formatting as I don't know how to post this any other way, lesson welcomed.
A quick (very) skim through hasn't pinpointed specific reference to my untrained eye as to its suitability for food use so have emailed for further confirmation, again will post accordingly.
I see that the actual manufacturer is cited, Atom Scientific. Might be an idea to contact them direct.
EDIT: not the manufacturer, they prepared the report, so probably won't be of much help.
Best wishes
Dave
Dave
-
Dave S
- Even further under the Table
- Posts: 2514
- Joined: Sun Apr 01, 2012 5:38 pm
- Location: Wirral, Merseyside
Re: Reducing alkalinity using acid.
I think this statement from the supplier, APCPure can lead us to the conclusion that the acid is food grade:
Phosphoric Acid 85% has a variety of industrial applications from rust removal, the acidification of food, and as an etching solution in dentistry and orthodontics.
Phosphoric Acid 85% has a variety of industrial applications from rust removal, the acidification of food, and as an etching solution in dentistry and orthodontics.
Best wishes
Dave
Dave
- orlando
- So far gone I'm on the way back again!
- Posts: 7201
- Joined: Thu Nov 17, 2011 3:22 pm
- Location: North Norfolk: Nearest breweries All Day Brewery, Salle. Panther, Reepham. Yetman's, Holt
Re: Reducing alkalinity using acid.
Finally the certificate of conformity confirming what we expected, it is food grade. So you can now order with peace of mind. Apologies again for poor formatting.
CERTIFICATE OF CONFORMITY
CERTCONF2011
This document certifies that the following product and specific batch has been manufactured to
and complies with the company’s standard Specification.
It further certifies that the batch has been tested by a diagnostic laboratory and complies with
the minimum specification for this product.
PRODUCT CODE GPC8046
DESCRIPTION Phosphoric Acid
BATCH NO Allocated at time of production
Manufacture Date 3 Year Shelf Life Expiry Date 3 Year Shelf Life
SPECIFICATION Phosphoric Acid 85% has a variety of industrial applications from rust removal, the acidifica tion of food, and as an etching solution in dentistry and orthodontics.
Minimum Assay: >85%
Maximum Level of Impurities:
Molecular Formula: H3PO4
Density: 1.885gm/ml
Melting Point: 42.35oC
Flash Point: Non Flammable
Disclaimer
The information contained herein is, to the best of our knowledge and belief, accurate. However, since conditions of handling and use are beyond our control, we do not guarantee any results, and we are not liable for any damage incurred by following these suggestions. Nothing contained herein is to be construed as a recommendation for use in violation of any patent or applicable laws or regulations.
TESTING
PROTOCOL
(WHERE APPLICABLE)
We confirm that this batch has been tested in our Laboratory and has been found to stain to our minimum standards and that sample will be retained for the life of the product
Peter Keenan
Commercial Director
Tested to our standard protocol.
CERTIFICATE OF CONFORMITY
CERTCONF2011
This document certifies that the following product and specific batch has been manufactured to
and complies with the company’s standard Specification.
It further certifies that the batch has been tested by a diagnostic laboratory and complies with
the minimum specification for this product.
PRODUCT CODE GPC8046
DESCRIPTION Phosphoric Acid
BATCH NO Allocated at time of production
Manufacture Date 3 Year Shelf Life Expiry Date 3 Year Shelf Life
SPECIFICATION Phosphoric Acid 85% has a variety of industrial applications from rust removal, the acidifica tion of food, and as an etching solution in dentistry and orthodontics.
Minimum Assay: >85%
Maximum Level of Impurities:
Molecular Formula: H3PO4
Density: 1.885gm/ml
Melting Point: 42.35oC
Flash Point: Non Flammable
Disclaimer
The information contained herein is, to the best of our knowledge and belief, accurate. However, since conditions of handling and use are beyond our control, we do not guarantee any results, and we are not liable for any damage incurred by following these suggestions. Nothing contained herein is to be construed as a recommendation for use in violation of any patent or applicable laws or regulations.
TESTING
PROTOCOL
(WHERE APPLICABLE)
We confirm that this batch has been tested in our Laboratory and has been found to stain to our minimum standards and that sample will be retained for the life of the product
Peter Keenan
Commercial Director
Tested to our standard protocol.
I am "The Little Red Brooster"
Fermenting:
Conditioning:
Drinking: Southwold Again,
Up Next: John Barleycorn (Barley Wine)
Planning: Winter drinking Beer
Fermenting:
Conditioning:
Drinking: Southwold Again,
Up Next: John Barleycorn (Barley Wine)
Planning: Winter drinking Beer
-
Dave S
- Even further under the Table
- Posts: 2514
- Joined: Sun Apr 01, 2012 5:38 pm
- Location: Wirral, Merseyside
Re: Reducing alkalinity using acid.
Good work Orlando. I see the shelf life is 3 years. I guess this will have a bearing on how much is ordered at a time. If you use say 20 ml per brew, that's 25 brews out of a 500 ml bottle. I could do that over about two years, but I'd be hard pushed to do 50 brews from 1 l over 3 years. I suppose some could be used for cleaning boiler elements etc. or, do a split order between 2 or 3 people. Either way, I'll get through the 250 ml bottle I bought from Brew UK first.
Best wishes
Dave
Dave
- orlando
- So far gone I'm on the way back again!
- Posts: 7201
- Joined: Thu Nov 17, 2011 3:22 pm
- Location: North Norfolk: Nearest breweries All Day Brewery, Salle. Panther, Reepham. Yetman's, Holt
Re: Reducing alkalinity using acid.
Yes really pleased to finally nail it down and have at least one other potential supplier via eBay. I brew about 25 times a year so will manage to do the 2 bottles over 3 years I'm fairly sure. Bloody good rust remover I understand too.Dave S wrote:Good work Orlando. I see the shelf life is 3 years. I guess this will have a bearing on how much is ordered at a time. If you use say 20 ml per brew, that's 25 brews out of a 500 ml bottle. I could do that over about two years, but I'd be hard pushed to do 50 brews from 1 l over 3 years. I suppose some could be used for cleaning boiler elements etc. or, do a split order between 2 or 3 people. Either way, I'll get through the 250 ml bottle I bought from Brew UK first.
I am "The Little Red Brooster"
Fermenting:
Conditioning:
Drinking: Southwold Again,
Up Next: John Barleycorn (Barley Wine)
Planning: Winter drinking Beer
Fermenting:
Conditioning:
Drinking: Southwold Again,
Up Next: John Barleycorn (Barley Wine)
Planning: Winter drinking Beer
-
Dave S
- Even further under the Table
- Posts: 2514
- Joined: Sun Apr 01, 2012 5:38 pm
- Location: Wirral, Merseyside
Re: Reducing alkalinity using acid.
Yeah, that's a point, doesn't have to all be used for brewing I suppose.orlando wrote:Yes really pleased to finally nail it down and have at least one other potential supplier via eBay. I brew about 25 times a year so will manage to do the 2 bottles over 3 years I'm fairly sure. Bloody good rust remover I understand too.Dave S wrote:Good work Orlando. I see the shelf life is 3 years. I guess this will have a bearing on how much is ordered at a time. If you use say 20 ml per brew, that's 25 brews out of a 500 ml bottle. I could do that over about two years, but I'd be hard pushed to do 50 brews from 1 l over 3 years. I suppose some could be used for cleaning boiler elements etc. or, do a split order between 2 or 3 people. Either way, I'll get through the 250 ml bottle I bought from Brew UK first.
Best wishes
Dave
Dave
- orlando
- So far gone I'm on the way back again!
- Posts: 7201
- Joined: Thu Nov 17, 2011 3:22 pm
- Location: North Norfolk: Nearest breweries All Day Brewery, Salle. Panther, Reepham. Yetman's, Holt
Re: Reducing alkalinity using acid.
Thought I would update this thread with a real life example of using Martin's calculator and checking it against a real brew using my new Voltcraft pH meter. I brewed a Bitter today and used the calculator to get the mash pH between 5.3 to 5.4. I also wanted to Burtonise the water so used the salt calculator as well as the mash & sparge acidification calculator. The calculator predicted the mash pH to be 5.38 and it came out at 5.34. The error margin could be accounted for by a process problem I had when my HLT element failed when I allowed the mash water to get too low so then had to decant from the HLT to boiler to get it up to temp then pump back to the HLT. My salt additions may not have "translated" well. Checked the final runnings and they were 5.37 so no risk of tannin extraction. So the next big test is the fermentation and how that goes and then of course the taste. Will report back in a month or so, but on the strength of today this part of the process looks to be shaping up nicely.
I am "The Little Red Brooster"
Fermenting:
Conditioning:
Drinking: Southwold Again,
Up Next: John Barleycorn (Barley Wine)
Planning: Winter drinking Beer
Fermenting:
Conditioning:
Drinking: Southwold Again,
Up Next: John Barleycorn (Barley Wine)
Planning: Winter drinking Beer
- orlando
- So far gone I'm on the way back again!
- Posts: 7201
- Joined: Thu Nov 17, 2011 3:22 pm
- Location: North Norfolk: Nearest breweries All Day Brewery, Salle. Panther, Reepham. Yetman's, Holt
Re: Reducing alkalinity using acid.
Another brewday another chance to use the mash pH calculator. This time an Amber style beer with an EBC of 13.2 so one of the more difficult styles with my tap water. I was astonished by how accurate it was predicting the mash pH.
This shows the summary of water treatment with the pH prediction.
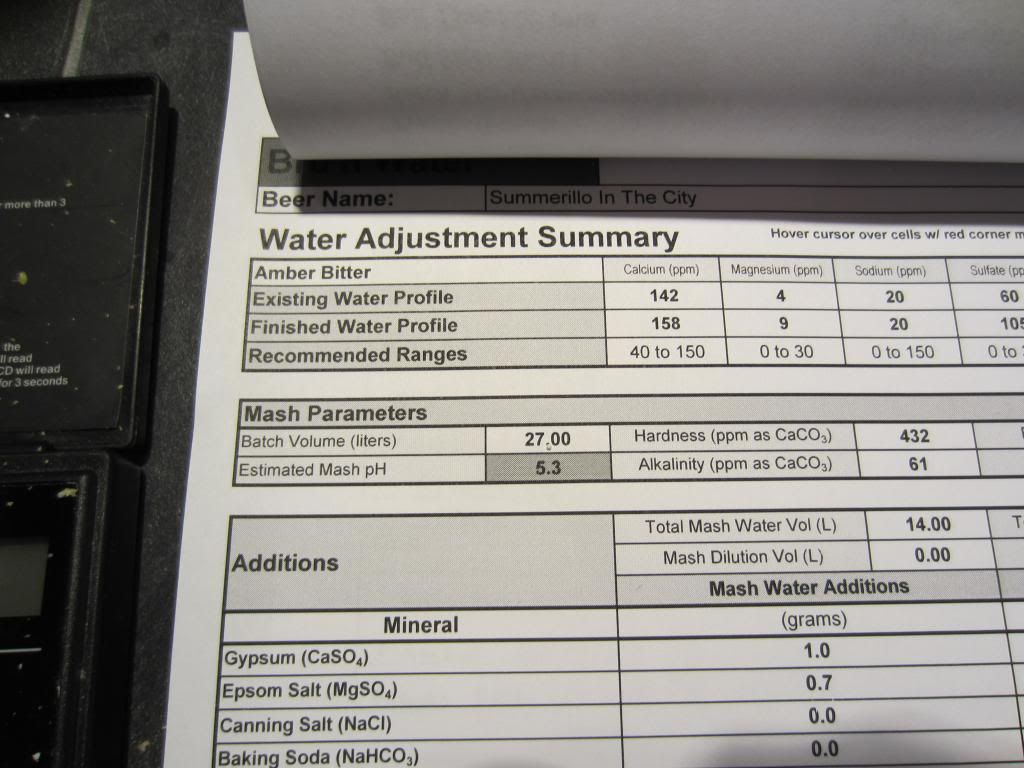
This shows the meter reading taken at just under 23c.

If it keeps this up I will be very pleased indeed, next stop of course is, has the beer improved? So far there are encouraging signs of fermentation's being a little more vigorous and a little quicker but this is too early and not enough examples to say it is due to the changes. I was disappointed to see that my sparge water was 6.2 rather than the 5.5 targeted but later realised I got my water volumes wrong and actually under acidified as a result. I checked the run off and to my surprise this only went up to 5.6 so hopefully won't have suffered from any excessive tannin extraction.
This shows the summary of water treatment with the pH prediction.

This shows the meter reading taken at just under 23c.

If it keeps this up I will be very pleased indeed, next stop of course is, has the beer improved? So far there are encouraging signs of fermentation's being a little more vigorous and a little quicker but this is too early and not enough examples to say it is due to the changes. I was disappointed to see that my sparge water was 6.2 rather than the 5.5 targeted but later realised I got my water volumes wrong and actually under acidified as a result. I checked the run off and to my surprise this only went up to 5.6 so hopefully won't have suffered from any excessive tannin extraction.
I am "The Little Red Brooster"
Fermenting:
Conditioning:
Drinking: Southwold Again,
Up Next: John Barleycorn (Barley Wine)
Planning: Winter drinking Beer
Fermenting:
Conditioning:
Drinking: Southwold Again,
Up Next: John Barleycorn (Barley Wine)
Planning: Winter drinking Beer
-
Dave S
- Even further under the Table
- Posts: 2514
- Joined: Sun Apr 01, 2012 5:38 pm
- Location: Wirral, Merseyside
Re: Reducing alkalinity using acid.
Glad to hear it's going well Orlando. I've only done one brew so far using MB's calculator. It's a Draught Bass clone. I didn't experience a faster ferment, in fact quite the opposite. It's been in the FV almost four weeks and is just getting ready for casking. It could be a temperature issue though. I used WLP023, Burton yeast which apparently likes it warm, and my room temp during fermentation was probably a bit low for it. The taste though is promising and it seems to be clearing well, though I'm intending to use auxiliary finings and isinglass in the cask. The pH readings came up bang on though, so I'm quite impressed.
The next brew is a week or so off. It's going to be a very hoppy IPA.
The next brew is a week or so off. It's going to be a very hoppy IPA.
Best wishes
Dave
Dave
- orlando
- So far gone I'm on the way back again!
- Posts: 7201
- Joined: Thu Nov 17, 2011 3:22 pm
- Location: North Norfolk: Nearest breweries All Day Brewery, Salle. Panther, Reepham. Yetman's, Holt
Re: Reducing alkalinity using acid.
Hi Dave, I reckon your right about temp being the issue, I doubt it could be anything else. I'm on my 4th brew with it and one of which I have started drinking and the first thing I have noticed is a really clean bitterness which is a little more than I like. What I mean is I can probably turn down the IBU's and get more bitterness perception for my hop than I've previously done. You may find this happens with your next planned brew. I have a Brown Ale on my brew list soon so it will be interesting to mash with the pH higher to suit this style and see if it will adjust the mash to the 5.4 to 5.5 range. If it does then it will be like using a thermometer to mash higher or lower and affect the outcome that way. I have a Burton style bitter, which incidentally only took 10 days, and has just finished (dry hopped yesterday with EKG pellets) and am looking forward to seeing how close I have got to that style using this calculator, all very promising.Dave S wrote:Glad to hear it's going well Orlando. I've only done one brew so far using MB's calculator. It's a Draught Bass clone. I didn't experience a faster ferment, in fact quite the opposite. It's been in the FV almost four weeks and is just getting ready for casking. It could be a temperature issue though. I used WLP023, Burton yeast which apparently likes it warm, and my room temp during fermentation was probably a bit low for it. The taste though is promising and it seems to be clearing well, though I'm intending to use auxiliary finings and isinglass in the cask. The pH readings came up bang on though, so I'm quite impressed.
The next brew is a week or so off. It's going to be a very hoppy IPA.
I am "The Little Red Brooster"
Fermenting:
Conditioning:
Drinking: Southwold Again,
Up Next: John Barleycorn (Barley Wine)
Planning: Winter drinking Beer
Fermenting:
Conditioning:
Drinking: Southwold Again,
Up Next: John Barleycorn (Barley Wine)
Planning: Winter drinking Beer
-
Dave S
- Even further under the Table
- Posts: 2514
- Joined: Sun Apr 01, 2012 5:38 pm
- Location: Wirral, Merseyside
Re: Reducing alkalinity using acid.
Yes I'm planning a brown ale and a porter soon. I'll probably bottle the brown, though the thought of that fills me with dread. The last time I bottled a brew was about 20 years ago, and remember all too well what a pain it is. But to do a good brown ale justice I think it will have to be done. Interesting what you say about the increased bitterness. The Bass tastes quite bitter but it's a bit early to tell yet if it's too much for my liking.orlando wrote:Hi Dave, I reckon your right about temp being the issue, I doubt it could be anything else. I'm on my 4th brew with it and one of which I have started drinking and the first thing I have noticed is a really clean bitterness which is a little more than I like. What I mean is I can probably turn down the IBU's and get more bitterness perception for my hop than I've previously done. You may find this happens with your next planned brew. I have a Brown Ale on my brew list soon so it will be interesting to mash with the pH higher to suit this style and see if it will adjust the mash to the 5.4 to 5.5 range. If it does then it will be like using a thermometer to mash higher or lower and affect the outcome that way. I have a Burton style bitter, which incidentally only took 10 days, and has just finished (dry hopped yesterday with EKG pellets) and am looking forward to seeing how close I have got to that style using this calculator, all very promising.Dave S wrote:Glad to hear it's going well Orlando. I've only done one brew so far using MB's calculator. It's a Draught Bass clone. I didn't experience a faster ferment, in fact quite the opposite. It's been in the FV almost four weeks and is just getting ready for casking. It could be a temperature issue though. I used WLP023, Burton yeast which apparently likes it warm, and my room temp during fermentation was probably a bit low for it. The taste though is promising and it seems to be clearing well, though I'm intending to use auxiliary finings and isinglass in the cask. The pH readings came up bang on though, so I'm quite impressed.
The next brew is a week or so off. It's going to be a very hoppy IPA.
Best wishes
Dave
Dave

