(That's water to the rest of us!) Beer is about 95% water, so if you want to discuss water treatment, filtering etc this is the place to do it!
-
JammyBStard
Post
by JammyBStard » Tue Aug 27, 2013 12:07 pm
I really like this spread sheet It's very user freindly, I got the full version from Martin a few days ago and have started trying to gather the info I need to make it work.
The problem as ever with water stuff is all the mixed terms used by the various parties, I've downloaded my water report and added the values into the input tab for starts: I think I have my values correct but the Cations and Anions don't tally and the Nitrate level is flagged.
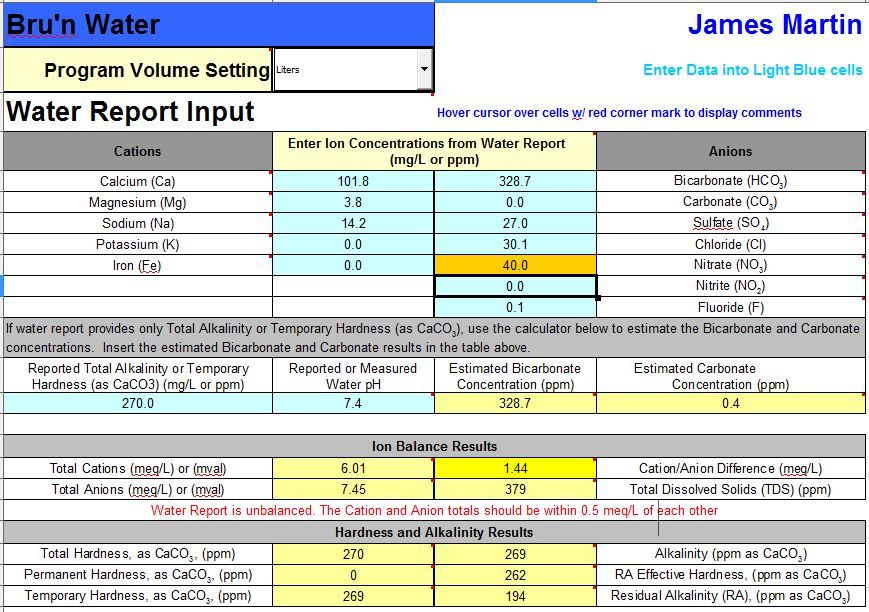
Can anyone shed any light on that for me?
-
WallyBrew
- Hollow Legs
- Posts: 476
- Joined: Sat Jan 05, 2008 11:30 pm
- Location: Surrey
Post
by WallyBrew » Tue Aug 27, 2013 12:46 pm
If the spreadsheet had any intelligence it would spot the 0 permanent hardness, recognise this as unlikely and therefore flag the bicarbonate as being wrong.
If you do not have an alkalinity testing kit, buy one and then enter a REAL value. While you are waiting for that you could try entering lower values of bicarbonate until it balances but then you still will not know what your alkalinity is.
As for why the nitrate is flagged - pass as I do not use this spreadsheet - perhaps it's warning you not to give this water to babies.
-
orlando
- So far gone I'm on the way back again!
- Posts: 7201
- Joined: Thu Nov 17, 2011 3:22 pm
- Location: North Norfolk: Nearest breweries All Day Brewery, Salle. Panther, Reepham. Yetman's, Holt
Post
by orlando » Tue Aug 27, 2013 1:05 pm
This is the reason:
"Nitrate is not a great concern in brewing, but should generally be less than 44 ppm in the water source to protect infants that may drink the water. Nitrate concentrations may also be reported in the form, Nitrate as Nitrogen (NO3-N). 44 ppm nitrate is equivalent to 10 ppm nitrate as nitrogen. Children and adults can tolerate higher nitrate concentration and the 44 ppm limit may not be a concern in brewing. Nitrate in brewing water should be less than 25 ppm (De Clerck, 1957). High nitrate concentration in the water may be converted to nitrite in the mash and nitrite becomes poisonous to yeast at levels above 0.1 ppm. If elevated nitrate levels are found in water, associated ions such as nitrite and ammonia should also be tested for."
Source
Brun' Water Knowledge.
It maybe because like me you live in an area that suffers from run off from artificial fertilisers that end up in the domestic supply. Mine is 30ppm and it flags anything over 25 ppm. A useful element of the spreadsheet is that where you see a little red triangle if you hover the cursor over it it gives a fuller explanation of what happens in the corresponding cell.
Maybe Wallybrew would share his superior calculator with us and we could avoid all the problems with Brun' Water

. How about it Wallybrew?

I am "The Little Red Brooster"
Fermenting:
Conditioning:
Drinking: Southwold Again,
Up Next: John Barleycorn (Barley Wine)
Planning: Winter drinking Beer
-
Matt12398
Post
by Matt12398 » Tue Aug 27, 2013 1:09 pm
That is a throw down if ever I heard one. I'll get the popcorn.
-
Aleman
- It's definitely Lock In Time
- Posts: 6132
- Joined: Sun Jun 03, 2007 11:56 am
- Location: Mashing In Blackpool, Lancashire, UK
Post
by Aleman » Tue Aug 27, 2013 1:48 pm
Why does it matter if the water report is unbalanced though? . . . obviously in this case there is an error (carbonate of 0), but really why do the anions and cations need to be within 0.5meq/L of each other??
The ONLY way you can be certain that you have a balanced liquor is if you are taking a FULL water analysis every time you are brewing, if you are relying on a water authority report, then it's extremely likely the values won't ever balance, as the readings for different ions are based on different numbers of readings taken at different times of the year. . . . Then what about the sum of the ions the calculator (whichever on it is) doesn't measure? We are interested in (anywhere) between 6 and 12 ions, but there are an awful lot more than that in the water that will affect the cation / anion balance.
I like Brun'water, but don't find the results to be wildly different from my own very simple hand cranked spreadsheet which does the job for me . . . although I might be very lucky with the starting liquor that I have that makes this so . . . I'm not offering up my spreadsheet as an alternative, as I can't be bothered to spend the time programming all the alerts and flags and checks and balances that Martin has put into Brun'water . . .
-
JammyBStard
Post
by JammyBStard » Tue Aug 27, 2013 2:18 pm
Thanks for the replies
orlando wrote:It maybe because like me you live in an area that suffers from run off from artificial fertilisers that end up in the domestic supply. Mine is 30ppm and it flags anything over 25 ppm. A useful element of the spreadsheet is that where you see a little red triangle if you hover the cursor over it it gives a fuller explanation of what happens in the corresponding cell:
I was thinking that perhaps the value was miss reported it been so high. But I live in heavily farmed chalk hills and our water is from bore holes so Orlando might have hit the nail on the head there. edit: that is the case I have since found a paper refering to it:
http://nora.nerc.ac.uk/3700/1/RR06004.pdf
My water report
http://www.ywonline.co.uk/web/WQZ.nsf/0 ... %20WSZ.pdf
dosent seem to have an figures for Carbonate or Total Alcalinity or Tempory Hardness, just Total hardness.
I must have got the worng figure for total Alkalinity and esimated the Bicarbonate incorrectly from there.
Can it be worked out from the water report? or will it have to be tested (And where do I gat a kit from)
-
orlando
- So far gone I'm on the way back again!
- Posts: 7201
- Joined: Thu Nov 17, 2011 3:22 pm
- Location: North Norfolk: Nearest breweries All Day Brewery, Salle. Panther, Reepham. Yetman's, Holt
Post
by orlando » Tue Aug 27, 2013 3:06 pm
Salifert do a cheap kit for testing alkalinity ebay will supply or you can go a little mad and get a
Hanna alkalinity meter. I have one and it is really simple to use but be aware that the presence of chlorine means it doesn't have utmost accuracy. They do sell the chlorine remover separately though.
I am "The Little Red Brooster"
Fermenting:
Conditioning:
Drinking: Southwold Again,
Up Next: John Barleycorn (Barley Wine)
Planning: Winter drinking Beer
-
WallyBrew
- Hollow Legs
- Posts: 476
- Joined: Sat Jan 05, 2008 11:30 pm
- Location: Surrey
Post
by WallyBrew » Tue Aug 27, 2013 3:15 pm
orlando wrote:
Maybe Wallybrew would share his superior calculator with us
I think I just did that - its the sort of lump in the head with the decaying neural network that keeps saying "brew beer, drink beer"
orlando wrote:This is the reason:
................ 44 ppm nitrate is equivalent to 10 ppm nitrate as nitrogen.Source
Brun' Water Knowledge.
Given that you appear to be a self confessed pedant then it should read: 44 ppm nitrate is equivalent to 10 ppm as nitrogen.
Once again the USA is out of step with the WHO.
EPA unless I've missed something (quite likely) you will see that it gives the nitrate (as NO3 not N) MCL as 10mg/L (or ppm) - again for the pedants the terms ppm and mg/L are not interchangeable.
WHO go to page 16
Is this all confusing and pointless?
Mr Brungard will be along shortly with an explanation ....................
-
orlando
- So far gone I'm on the way back again!
- Posts: 7201
- Joined: Thu Nov 17, 2011 3:22 pm
- Location: North Norfolk: Nearest breweries All Day Brewery, Salle. Panther, Reepham. Yetman's, Holt
Post
by orlando » Tue Aug 27, 2013 3:29 pm
WallyBrew wrote:
Given that you appear to be a self confessed pedant then it should read: 44 ppm nitrate is equivalent to 10 ppm as nitrogen.
Once again the USA is out of step with the WHO.
EPA unless I've missed something (quite likely) you will see that it gives the nitrate (as NO3 not N) MCL as 10mg/L (or ppm) - again for the pedants the terms ppm and mg/L are not interchangeable.
WHO go to page 16
Is this all confusing and pointless?
Mr Brungard will be along shortly with an explanation ....................
Hopefully, certainly more polite.

I am "The Little Red Brooster"
Fermenting:
Conditioning:
Drinking: Southwold Again,
Up Next: John Barleycorn (Barley Wine)
Planning: Winter drinking Beer
-
WallyBrew
- Hollow Legs
- Posts: 476
- Joined: Sat Jan 05, 2008 11:30 pm
- Location: Surrey
Post
by WallyBrew » Tue Aug 27, 2013 3:40 pm
orlando wrote:
Hopefully, certainly more polite.

Sorry - was I impolite at some point or just stating fact
-
orlando
- So far gone I'm on the way back again!
- Posts: 7201
- Joined: Thu Nov 17, 2011 3:22 pm
- Location: North Norfolk: Nearest breweries All Day Brewery, Salle. Panther, Reepham. Yetman's, Holt
Post
by orlando » Tue Aug 27, 2013 3:43 pm
WallyBrew wrote:orlando wrote:
Hopefully, certainly more polite.

Sorry - was I impolite at some point or just stating fact
Alarmingly close

I am "The Little Red Brooster"
Fermenting:
Conditioning:
Drinking: Southwold Again,
Up Next: John Barleycorn (Barley Wine)
Planning: Winter drinking Beer
-
JammyBStard
Post
by JammyBStard » Tue Aug 27, 2013 3:47 pm
-
orlando
- So far gone I'm on the way back again!
- Posts: 7201
- Joined: Thu Nov 17, 2011 3:22 pm
- Location: North Norfolk: Nearest breweries All Day Brewery, Salle. Panther, Reepham. Yetman's, Holt
Post
by orlando » Tue Aug 27, 2013 3:53 pm
I am "The Little Red Brooster"
Fermenting:
Conditioning:
Drinking: Southwold Again,
Up Next: John Barleycorn (Barley Wine)
Planning: Winter drinking Beer
-
mabrungard
- Piss Artist
- Posts: 250
- Joined: Sat Dec 15, 2012 3:17 pm
- Location: Indianapolis, Indiana
Post
by mabrungard » Tue Aug 27, 2013 7:09 pm
A balance in the cations and anions is one of the best ways of providing a check on the quality of the data. Since the water input screen of Bru'n Water enables you to put in all the MAJOR cations and anions that many potable water supplies will contain, you will be able to show the ions balancing to some degree (assuming you have data on all those ions). Sure, there are other ions that may be present in water such as arsenic, aluminum, manganese, silicate, etc. But they are typically present at such low concentration that including or excluding them from the ion totals won't change the balance much.
In the case of the water that Jammy presents, all the major ions excepting alkalinity or the carbonate species are presented. The difference in the ion totals is significant and should raise a concern. Fortunately since the data from the water report is fairly complete and the water quality doesn't seem to vary too much, it does appear that a brewer could 'back-calculate' the bicarbonate content of the water by finding the concentration that balances the ion totals. Performing testing on the water to confirm the alkalinity or bicarbonate values would be a wise double-check.
To my knowledge, USEPA and WHO are in agreement on the nitrate issue. USEPA presents the limit as 10 ppm (nitrate as N) which is roughly 44 ppm as nitrate. That is in place for drinking water primarily to protect infants. I'm not quite sure why I have the program flagging the nitrate level at anything less than 10 ppm, but that will be revised to 44 ppm. For brewing, even the 44 ppm level is not really a big deal.
-
keith1664
- Lost in an Alcoholic Haze
- Posts: 640
- Joined: Mon Oct 04, 2010 8:54 pm
- Location: Norwich
Post
by keith1664 » Tue Aug 27, 2013 9:43 pm
orlando wrote:Salifert do a cheap kit for testing alkalinity ebay will supply or you can go a little mad and get a
Hanna alkalinity meter. I have one and it is really simple to use but be aware that the presence of chlorine means it doesn't have utmost accuracy. They do sell the chlorine remover separately though.
Any idea what the chlorine remover is and could you use sodium metabisulphite instead?
In or near Norwich? Interested in meeting up monthly to talk and drink beer? PM me for details.


