Strange Salifert Alkalinity behaviour
-
will_raymo2000
Strange Salifert Alkalinity behaviour
Hi all,
Brewed yesterday, 80L total water, 40L ashbeck and 40L tap water (tested at near 300ppm alkalinity).
I added 40ml CRS to treat the tapwater part of the water and then went to test with salifert again but it was being very strange and turning the pink colour as soon as I added even 1 drop of the indicator dye. With the ashbeck alone and the tap water alone it was fine and was the green colour but this mixture and the CRS caused the dye to change immediately.
The pH measured with test strips was about 5.4.
Any ideas what this means?
Brewed yesterday, 80L total water, 40L ashbeck and 40L tap water (tested at near 300ppm alkalinity).
I added 40ml CRS to treat the tapwater part of the water and then went to test with salifert again but it was being very strange and turning the pink colour as soon as I added even 1 drop of the indicator dye. With the ashbeck alone and the tap water alone it was fine and was the green colour but this mixture and the CRS caused the dye to change immediately.
The pH measured with test strips was about 5.4.
Any ideas what this means?
-
Mr. Dripping
Re: Strange Salifert Alkalinity behaviour
Most likely you've added too much CRS and taken out all of the alkalinity.
- Eric
- Even further under the Table
- Posts: 2918
- Joined: Fri Mar 13, 2009 1:18 am
- Location: Sunderland.
Re: Strange Salifert Alkalinity behaviour
From what you've reported, that shouldn't be.
The indicator in the Salifert kit goes pink at about pH 4.5 when the last vestiges of alkalinity are assumed to have been neutralised, so that pH measurement conflicts.
I can only think insufficient time was allowed for the acid to do its job and the CO2 fully liberated before the test was done else your water has much less alkalinity.
The graph is of a test done recently on a litre of my tap water with CRS. The actual end point for alkalinity is at the inflection point (where the curve changes direction, the steepest bit) rather than a particular value of pH, but in practice the two are close enough that if you think you need to find its actually value, you've forgotten the object is to brew some beer.
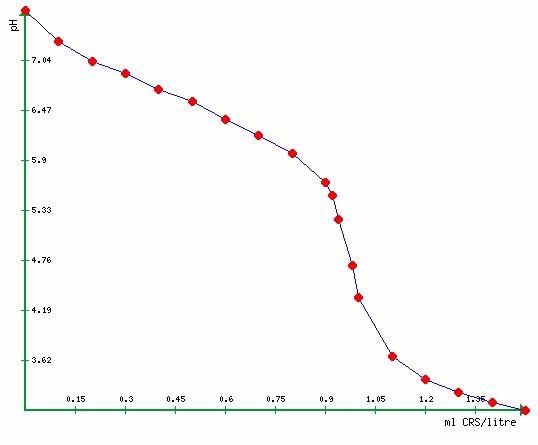
The indicator in the Salifert kit goes pink at about pH 4.5 when the last vestiges of alkalinity are assumed to have been neutralised, so that pH measurement conflicts.
I can only think insufficient time was allowed for the acid to do its job and the CO2 fully liberated before the test was done else your water has much less alkalinity.
The graph is of a test done recently on a litre of my tap water with CRS. The actual end point for alkalinity is at the inflection point (where the curve changes direction, the steepest bit) rather than a particular value of pH, but in practice the two are close enough that if you think you need to find its actually value, you've forgotten the object is to brew some beer.

Without patience, life becomes difficult and the sooner it's finished, the better.
-
will_raymo2000
Re: Strange Salifert Alkalinity behaviour
I'm hoping its not the former option and I somehow wrongly dosed in CRS.. What would happen to the beer if I brewed it with no alkalinity at all? The brew went better than expected and my efficiency was higher than it has been in the past and I can't tell any massive off tastes in the unfermented wort but I suppose I shall have to wait and see.
- Eric
- Even further under the Table
- Posts: 2918
- Joined: Fri Mar 13, 2009 1:18 am
- Location: Sunderland.
Re: Strange Salifert Alkalinity behaviour
You wouldn't be the first to make or drink such a beer. The yeast could have a few rough days.
Can you check how much CRS you did use then do a small scale test with a similar mix of waters and pro rata CRS?
You didn't perhaps use the same syringe for dosing with CRS as collected the 4ml sample for the Salifert test? Any small residue of acid in the syringe would cause the reading you found.
Can you check how much CRS you did use then do a small scale test with a similar mix of waters and pro rata CRS?
You didn't perhaps use the same syringe for dosing with CRS as collected the 4ml sample for the Salifert test? Any small residue of acid in the syringe would cause the reading you found.
Without patience, life becomes difficult and the sooner it's finished, the better.
-
will_raymo2000
Re: Strange Salifert Alkalinity behaviour
No way of actually knowing what I added as I measured the amount into a jug using a set of scales. I now realise that CRS is not the same density as water therefore i'd be wrong using the 1g = 1ml assumption I was using.
I think I'll have to put this down to experience and hope I get something nice at the end of it. It is a belgian style after all and a bit of stressed yeast probably isn't such a bad thing (??). There was a nice krausen on it less than 6 hours after pitching too.
I think I'll have to put this down to experience and hope I get something nice at the end of it. It is a belgian style after all and a bit of stressed yeast probably isn't such a bad thing (??). There was a nice krausen on it less than 6 hours after pitching too.
- Eric
- Even further under the Table
- Posts: 2918
- Joined: Fri Mar 13, 2009 1:18 am
- Location: Sunderland.
Re: Strange Salifert Alkalinity behaviour
The SG of CRS is greater than that of water so 40g has a lesser volume than 40ml and thereby less reduction in alkalinity.
Thaking Murphy's SG the volume would have been 36.9ml producing a total reduction in alkalinity of about 6750mg CaCO3. If your water was anything like you measured that would not have been an excesive dose.
Time for a few more tests?
Thaking Murphy's SG the volume would have been 36.9ml producing a total reduction in alkalinity of about 6750mg CaCO3. If your water was anything like you measured that would not have been an excesive dose.
Time for a few more tests?
Without patience, life becomes difficult and the sooner it's finished, the better.
-
wilfh
- Piss Artist
- Posts: 295
- Joined: Sat Jun 30, 2012 4:09 pm
- Location: Half way between Newcastle and Sunderland
Re: Strange Salifert Alkalinity behaviour
I've put too much crs in ad well . Beer wasn't the best but has aged out ok. Still drinkable but with a. Mineral bite
-
Charles1968
Re: Strange Salifert Alkalinity behaviour
Agree with Eric - see if you can repeat the result. I think you won't be able to, as something isn't right. Did you stir the liquor thoroughly? Was the test container or syringe contaminated with CRS? Maybe check the alkalinity of your tap water again.will_raymo2000 wrote:Hi all,
Brewed yesterday, 80L total water, 40L ashbeck and 40L tap water (tested at near 300ppm alkalinity).
I added 40ml CRS to treat the tapwater part of the water and then went to test with salifert again but it was being very strange and turning the pink colour as soon as I added even 1 drop of the indicator dye. With the ashbeck alone and the tap water alone it was fine and was the green colour but this mixture and the CRS caused the dye to change immediately.
The pH measured with test strips was about 5.4.
Any ideas what this means?

