Mash ph
-
andyisavinit
Mash ph
I'm 30mins into my mash as i type.
I adjusted my water to ph 5.8 with lactic acid (7ml) before adding my grain expecting it to lower even more to about 5.4 ish.
After adding my grain it's gone up to 6.2 not down.
4400g marris otter
440g crystal
240g torrifed wheat
marris otter grain is over 6 months old
Why is my ph going up? I tested 5mins, then 10, then 15 then 20 mins after mash in. It didn't really change. So i added more lactic acid (2ml ish) and have settled at 5.8ph as couldn't be arsed messing around anymore.
I adjusted my water to ph 5.8 with lactic acid (7ml) before adding my grain expecting it to lower even more to about 5.4 ish.
After adding my grain it's gone up to 6.2 not down.
4400g marris otter
440g crystal
240g torrifed wheat
marris otter grain is over 6 months old
Why is my ph going up? I tested 5mins, then 10, then 15 then 20 mins after mash in. It didn't really change. So i added more lactic acid (2ml ish) and have settled at 5.8ph as couldn't be arsed messing around anymore.
- orlando
- So far gone I'm on the way back again!
- Posts: 7201
- Joined: Thu Nov 17, 2011 3:22 pm
- Location: North Norfolk: Nearest breweries All Day Brewery, Salle. Panther, Reepham. Yetman's, Holt
Re: Mash ph
Did you let the sample cool to room temp? I put mine in a small vessel and then into cold water to speed things up. It can also take a while for the measurement to settle, wait, are you using strips or an electronic device? What was your alkalinity before and after using the acid?andyisavinit wrote:I'm 30mins into my mash as i type.
I adjusted my water to ph 5.8 with lactic acid (7ml) before adding my grain expecting it to lower even more to about 5.4 ish.
After adding my grain it's gone up to 6.2 not down.
4400g marris otter
440g crystal
240g torrifed wheat
marris otter grain is over 6 months old
Why is my ph going up? I tested 5mins, then 10, then 15 then 20 mins after mash in. It didn't really change. So i added more lactic acid (2ml ish) and have settled at 5.8ph as couldn't be arsed messing around anymore.
I am "The Little Red Brooster"
Fermenting:
Conditioning:
Drinking: Southwold Again,
Up Next: John Barleycorn (Barley Wine)
Planning: Winter drinking Beer
Fermenting:
Conditioning:
Drinking: Southwold Again,
Up Next: John Barleycorn (Barley Wine)
Planning: Winter drinking Beer
-
AnthonyUK
Re: Mash ph
The pH of the water is not what you need to know but how much buffering capacity it has.
Are you using any software to assist you in working it out and do you have a detailed report of your water composition?
Are you using any software to assist you in working it out and do you have a detailed report of your water composition?
Re: Mash ph
The pH of your water is irrelevant. Did you test your alkalinity (not pH) using a salifert kit? Have a look here
-
andyisavinit
Re: Mash ph
Thanks for your replies - I'm now 20mins into the boil.
Yes have checked with salifert, yes using a electronic ph tester. Yes cooled samples. I understand the buffering concept (well i think i do)
I use brunwater to adjust my water too.
In this batch i added 2g of gypsum to the grain and 2g of calcium chloride to the mash (just after mash in). As well as the 9ml of lactic acid pre mash in.
My water if very high in caco3 - 162 ish. So i boil my water the night before and this brings it down to approx 35-70. I didn't actually test it this time after the boil. I did test before boiling and it was the usual high 160's so didn't bother testing after boil, working on experience.
This is what really happened, i was a bit brief earlier:
Bru'n water told me 7.5 ml of acid. I added 7.5 and it was still too high, I was adding in acid in 2-3 ml increments to be safe and it was adjusting the ph by a very little. I know the calculators are a guide. So when i reached 7.5 ml and it was still too high (maybe 5.9) so i added another 2ml. Then the next reading was 4.6-4.8. WHAT!!! So i thought i'll take 2l of water out and add in 2l of tap water (high in alkilinty) to bring it back up. I don't have any chalk. It did - good! Reading = 5.8.
So now i thought when i add grain the ph will come down a bit and i'll hit my 5.4 mark. Or near enough. But NO - PH gone up to 6.2!!!! So had to add more acid to get it back down - really really annoying after messing about before hand. I don't get it.
My ph tester is accurate - well it behaves as expected most of the time! I waited long enough for mash ph stabilisation. Grrr
Any thoughts. My thoughts are that when adding lactic, add it very slowly when you are getting near to your target.
With my grain bill brunwater (just tested) says the ph will drop by 0.1 - less than i thought, guess thats because theres no dark grains
Yes have checked with salifert, yes using a electronic ph tester. Yes cooled samples. I understand the buffering concept (well i think i do)
I use brunwater to adjust my water too.
In this batch i added 2g of gypsum to the grain and 2g of calcium chloride to the mash (just after mash in). As well as the 9ml of lactic acid pre mash in.
My water if very high in caco3 - 162 ish. So i boil my water the night before and this brings it down to approx 35-70. I didn't actually test it this time after the boil. I did test before boiling and it was the usual high 160's so didn't bother testing after boil, working on experience.
This is what really happened, i was a bit brief earlier:
Bru'n water told me 7.5 ml of acid. I added 7.5 and it was still too high, I was adding in acid in 2-3 ml increments to be safe and it was adjusting the ph by a very little. I know the calculators are a guide. So when i reached 7.5 ml and it was still too high (maybe 5.9) so i added another 2ml. Then the next reading was 4.6-4.8. WHAT!!! So i thought i'll take 2l of water out and add in 2l of tap water (high in alkilinty) to bring it back up. I don't have any chalk. It did - good! Reading = 5.8.
So now i thought when i add grain the ph will come down a bit and i'll hit my 5.4 mark. Or near enough. But NO - PH gone up to 6.2!!!! So had to add more acid to get it back down - really really annoying after messing about before hand. I don't get it.
My ph tester is accurate - well it behaves as expected most of the time! I waited long enough for mash ph stabilisation. Grrr
Any thoughts. My thoughts are that when adding lactic, add it very slowly when you are getting near to your target.
With my grain bill brunwater (just tested) says the ph will drop by 0.1 - less than i thought, guess thats because theres no dark grains
-
Piscator
Re: Mash ph
Dont confuse Alkalinity with Alkali - they are 2 different things.andyisavinit wrote: So i thought i'll take 2l of water out and add in 2l of tap water (high in alkilinty) to bring it back up.
Put the pH meter away and use the salifert kit to check alkalinity when you make your lactic additions and don't forget to give it enough time for the CO2 evolved to dissipate. The pH of the mash liquor has no direct bearing on the mash pH which is driven by reactions taking place within the mash which dictate the overall pH - it is NOT set by the liquor pH. Excess alkalinity is the enemy here as it resists the changes in mash pH preventing you reaching your target.
Cheers
Steve
-
andyisavinit
Re: Mash ph
Ok steve thanks. I'll step away from the meter.
I thought i had a handle on this chemistry murlarky but obviously not! I will do some recapping on mash and liquor ph and alkalinity. Hope i've not wasted everyones time, i feel a bit of a noob again. I'm doing all the testing and using additions and getting information/figures and then don't know what to do with it/misinterpreting it.
Back to the chemistry reading.
It was a hoppy nelson sauvin ale - sure it'll be very drinkable whatever happens.
I thought i had a handle on this chemistry murlarky but obviously not! I will do some recapping on mash and liquor ph and alkalinity. Hope i've not wasted everyones time, i feel a bit of a noob again. I'm doing all the testing and using additions and getting information/figures and then don't know what to do with it/misinterpreting it.
Back to the chemistry reading.
It was a hoppy nelson sauvin ale - sure it'll be very drinkable whatever happens.
Re: Mash ph
Read the link I posted. Then if you have any questions, post them in the brewing liquor section
-
Piscator
Re: Mash ph
Andy don't worry about it, it's part of the fun!
In your shoes I would leave the "flavour" ions alone for the time being and work on understanding alkalinity - you have a salifert kit so have the necessary equipment to make informed adjustments. Measure the alkalinity and work out the quantity of acid to use to get to the level you need for the style you are brewing - make your acid addition and re-test with the salifert kit to check you are in the right ballpark.
Next - brew beer
If the alkalinity is right for the style the mash pH should look after itself.
Once you have this sorted move to adjusting calcium, sulphate and chloride as necessary if you have decent information about your water supply.
Most of all though, enjoy your beer
Cheers
Steve
In your shoes I would leave the "flavour" ions alone for the time being and work on understanding alkalinity - you have a salifert kit so have the necessary equipment to make informed adjustments. Measure the alkalinity and work out the quantity of acid to use to get to the level you need for the style you are brewing - make your acid addition and re-test with the salifert kit to check you are in the right ballpark.
Next - brew beer
If the alkalinity is right for the style the mash pH should look after itself.
Once you have this sorted move to adjusting calcium, sulphate and chloride as necessary if you have decent information about your water supply.
Most of all though, enjoy your beer
Cheers
Steve
- orlando
- So far gone I'm on the way back again!
- Posts: 7201
- Joined: Thu Nov 17, 2011 3:22 pm
- Location: North Norfolk: Nearest breweries All Day Brewery, Salle. Panther, Reepham. Yetman's, Holt
Re: Mash ph
Without wishing to make things more complex and confusing it is also worth considering one other thing when adjusting alkalinity for darker beers like stouts and porters. These beers use grains that have a natural acidifying effect on the wort pH so don't need as much acid in your mash liquour. However if you are sparging your grains it is important to adjust your sparge liquour to the 20-30 range as you would for all the liquour when making a pale. This is because high alkalinity can extract unwanted tannins in the wort, amongst other undesirable things caused by high alkalinity in your water.
I am "The Little Red Brooster"
Fermenting:
Conditioning:
Drinking: Southwold Again,
Up Next: John Barleycorn (Barley Wine)
Planning: Winter drinking Beer
Fermenting:
Conditioning:
Drinking: Southwold Again,
Up Next: John Barleycorn (Barley Wine)
Planning: Winter drinking Beer
-
BenB
Re: Mash ph
Acidifying sparge water is simple enough but recently I started wondering what happens to flavour with an overly acidic beer? Not an acidic mash but an acidic end result perhaps from over acidifying the sparge water? I treat all my water with crs and wonder if I'm over acidifying the beer. Can you measure beer pH with a meter and do you have to degas first?
- Aleman
- It's definitely Lock In Time
- Posts: 6132
- Joined: Sun Jun 03, 2007 11:56 am
- Location: Mashing In Blackpool, Lancashire, UK
Re: Mash ph
If you are using the acid to neutralise alkalinity to a level you desire from one you have measured then you do not over acidify the beer If you are just adding acid at random based on a potentially out of date value on a water report you run the risk. This is especially true if you add all the acid in one go without checking, it has been known for the acid to be stronger that claimed!
Even if you just acidify your sparge liquor to pH 5.8 you are unlikely to be over acidifying your beer as at pH 5.8 there is still some alkalinity present (The 'test' for alkalinity is to add acid until you hit the methyl orange end point ??? ~4.3 IIRC, and that is Zero alkalinity).
I find that if you get your mash pH right then you don't have to worry about what the pH is later in the process, it always seems to fall in the right region. Yes you can measure beer pH with a meter, and no you don't need to degas first once it has fermented.
Even if you just acidify your sparge liquor to pH 5.8 you are unlikely to be over acidifying your beer as at pH 5.8 there is still some alkalinity present (The 'test' for alkalinity is to add acid until you hit the methyl orange end point ??? ~4.3 IIRC, and that is Zero alkalinity).
I find that if you get your mash pH right then you don't have to worry about what the pH is later in the process, it always seems to fall in the right region. Yes you can measure beer pH with a meter, and no you don't need to degas first once it has fermented.
- Eric
- Even further under the Table
- Posts: 2920
- Joined: Fri Mar 13, 2009 1:18 am
- Location: Sunderland.
Re: Mash ph
Beer after fermentation usually has a pH of between 3.7 and 4.2. My beers are most often between pH 4.0 and pH 4.2.
Below is a graph of pH in a 1 litre sample of my water supply progressively treated with CRS.
1ml of CRS is thought to neutral 183mg of CaCO3.
The red dots show the measurements taken, then joined by straight lines for convienience.
pH does not measure alkalinity, even the end point varies slightly.
In a test done last November on my tap water the end point for alkalinity was determined to be at pH 4.35, alkalinity at pH 5.65 was 15.3mg/l CaCO3 and at pH 5.98 was 33.6 mg/l CaCO3. You cannot assume that because I got these results that your water will have similar values and nor can I assume the same would apply to my water the next day or the day previous or any other day.
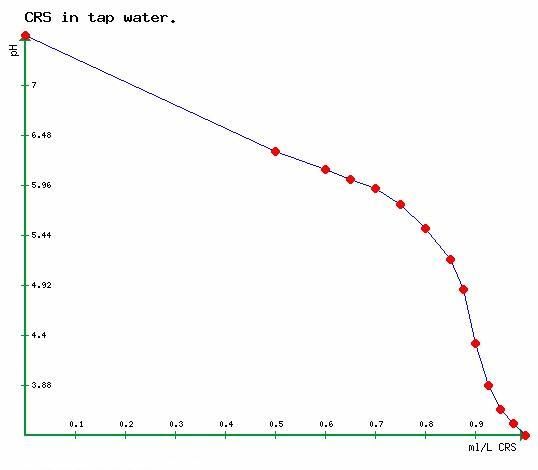
Below is a graph of pH in a 1 litre sample of my water supply progressively treated with CRS.
1ml of CRS is thought to neutral 183mg of CaCO3.
The red dots show the measurements taken, then joined by straight lines for convienience.
pH does not measure alkalinity, even the end point varies slightly.
In a test done last November on my tap water the end point for alkalinity was determined to be at pH 4.35, alkalinity at pH 5.65 was 15.3mg/l CaCO3 and at pH 5.98 was 33.6 mg/l CaCO3. You cannot assume that because I got these results that your water will have similar values and nor can I assume the same would apply to my water the next day or the day previous or any other day.

Without patience, life becomes difficult and the sooner it's finished, the better.
- mabrungard
- Piss Artist
- Posts: 250
- Joined: Sat Dec 15, 2012 3:17 pm
- Location: Indianapolis, Indiana
Re: Mash ph
andy, I am assuming that you were adding acid directly into the mash and stirring? Under that condition, it is very easy to overdose the surficial zone of your mash bed and that can be reflected by a low pH. While the information regarding the exhaustion of alkalinity during a titration is quite valid, I'm not positive that this is the phenomena that led to your apparent problem.
Was the mash VERY thoroughly mixed following those acid additions? Is this a RIMS or HERMS with a good wort recirculation? Did you collect and test a wort sample from deep within your mash bed? If all of these answers are Yes, then it is likely that you exhausted the mash alkalinity and the pH plummeted. If not, it is possible that your pH reading was not representative of the entire mash and wort volume.
Sorry for your troubles.
Was the mash VERY thoroughly mixed following those acid additions? Is this a RIMS or HERMS with a good wort recirculation? Did you collect and test a wort sample from deep within your mash bed? If all of these answers are Yes, then it is likely that you exhausted the mash alkalinity and the pH plummeted. If not, it is possible that your pH reading was not representative of the entire mash and wort volume.
Sorry for your troubles.
Martin B
Indianapolis, Indiana
BJCP National Judge
Foam Blowers of Indiana (FBI)
Brewing Water Information at: https://www.brunwater.com/
Like Bru'n Water on Facebook for occasional discussions on brewing water and Bru'n Water
https://www.facebook.com/pages/Brun-Wat ... =bookmarks
Indianapolis, Indiana
BJCP National Judge
Foam Blowers of Indiana (FBI)
Brewing Water Information at: https://www.brunwater.com/
Like Bru'n Water on Facebook for occasional discussions on brewing water and Bru'n Water
https://www.facebook.com/pages/Brun-Wat ... =bookmarks

