Bedford water
Re: Bedford water
Hi guys im brewing a pale ale at the weekend and wanted some advise on water treatment. I have used crs in tge past but am sure its giving an offtaste to the finished beer!

Re: Bedford water
Anyone else brew with bedford water who could help?
- Eric
- Even further under the Table
- Posts: 2919
- Joined: Fri Mar 13, 2009 1:18 am
- Location: Sunderland.
Re: Bedford water
How much CRS and how did you determine the quantity?
Without patience, life becomes difficult and the sooner it's finished, the better.
Re: Bedford water
I used 31 mls of crs for 38 litres of water i used a salifert kit to determine the alkilinity.
I was thinking about boiling the water to reduce the alkilinity.
I was thinking about boiling the water to reduce the alkilinity.
- jmc
- Even further under the Table
- Posts: 2486
- Joined: Thu May 13, 2010 11:43 pm
- Location: Swaledale, North Yorkshire
Re: Bedford water
Hi timbobist. I'm not too far away in Toddington.
Really hard water around here.
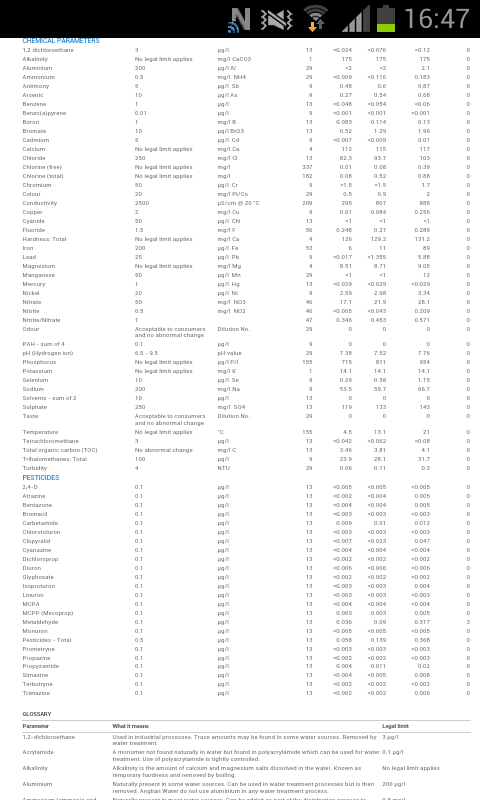
My report says my water hardness as Calcium carbonate (mg/l) is 303.25
For water treatment I use campden tablets (in solution) and CRS plus gypsum, calcium chloride and epsom salts as suggested by Graham's Water Treatment Calculator on Jims
Your 31 mls of crs for 38 litres of water is ~ 0.82mls/L
If I plug my local water figures intoGraham's Water Treatment Calculator for a Dry Pale Ale, I get 0.81ml/L.
It sounds like your maths is OK as I suspect you water info is very close to mine.
However when using ph papers ( I know they're only approximate) I've been getting consistently low-end pH of 5.2 or possibly lower.
I've experimented with a couple of brews recently and used 60% of suggested CRS and pH has been Ok ~5.2 - 5.5
Really hard water around here.
My report says my water hardness as Calcium carbonate (mg/l) is 303.25
For water treatment I use campden tablets (in solution) and CRS plus gypsum, calcium chloride and epsom salts as suggested by Graham's Water Treatment Calculator on Jims
Your 31 mls of crs for 38 litres of water is ~ 0.82mls/L
If I plug my local water figures intoGraham's Water Treatment Calculator for a Dry Pale Ale, I get 0.81ml/L.
It sounds like your maths is OK as I suspect you water info is very close to mine.
However when using ph papers ( I know they're only approximate) I've been getting consistently low-end pH of 5.2 or possibly lower.
I've experimented with a couple of brews recently and used 60% of suggested CRS and pH has been Ok ~5.2 - 5.5
- Eric
- Even further under the Table
- Posts: 2919
- Joined: Fri Mar 13, 2009 1:18 am
- Location: Sunderland.
Re: Bedford water
Then I don't see how CRS would be the problem if your measurements are correct or you tested residual alkalinity.
While you can't generally rely on such water reports, your findings would confirm it isn't wildly out and I would have thought CRS would produce a usable profile for a range of beers, including pale ales.
Boiling and decanting off deposited CaCO3 would reduce CRS needed to allow a larger range of bias to sulphate or chloride, but that is more to do with preference than quality.
If I've read the report correctly there's a lot more sodium than I've ever experienced, but I'm not convinced that would be the problem nor could boiling help.
Sorry, can't help really. I would have thought CRS alone, mashing 4Kg pale malt for a 23 litre brew boiled for 90 minutes with a balancing bittering hop and a couple of handfuls of your favourite aroma hop at the end would with virtually any yeast have you sitting in seventh heaven all summer long.
While you can't generally rely on such water reports, your findings would confirm it isn't wildly out and I would have thought CRS would produce a usable profile for a range of beers, including pale ales.
Boiling and decanting off deposited CaCO3 would reduce CRS needed to allow a larger range of bias to sulphate or chloride, but that is more to do with preference than quality.
If I've read the report correctly there's a lot more sodium than I've ever experienced, but I'm not convinced that would be the problem nor could boiling help.
Sorry, can't help really. I would have thought CRS alone, mashing 4Kg pale malt for a 23 litre brew boiled for 90 minutes with a balancing bittering hop and a couple of handfuls of your favourite aroma hop at the end would with virtually any yeast have you sitting in seventh heaven all summer long.
Without patience, life becomes difficult and the sooner it's finished, the better.
Re: Bedford water
Thanks for the replies guys. Ive just been reading a thread called anglian water on jims and a guy on there suggests that with the already high chloride in the water crs would not be ideal. Looking at his water profile it is quite similar to mine he only live 10 - 15 miles away. Maybe that is the taste?
- Eric
- Even further under the Table
- Posts: 2919
- Joined: Fri Mar 13, 2009 1:18 am
- Location: Sunderland.
Re: Bedford water
That taste would normally be maltiness and a touch of residual sweetness. I have a similar profile here but prefer sweet pales to dry.
However, you are correct in saying that using CRS will produce a profile with your water that probably can't be Burtonised as you won't be able to get a large enough ratio of sulphates to chloride, but there's more than one way of skinning a cat.
If I want a drier beer I use Nottingham yeast, mash cooler and/or a little longer and substitute a little white sugar for malt. There are others too, but whatever you try, good luck.
Eric.
However, you are correct in saying that using CRS will produce a profile with your water that probably can't be Burtonised as you won't be able to get a large enough ratio of sulphates to chloride, but there's more than one way of skinning a cat.
If I want a drier beer I use Nottingham yeast, mash cooler and/or a little longer and substitute a little white sugar for malt. There are others too, but whatever you try, good luck.
Eric.
Without patience, life becomes difficult and the sooner it's finished, the better.
-
Matt12398
Re: Bedford water
Have you considered using a different acid to reduce your carbonate such as phosphoric. It won't affect your sulphate levels in the same way CRS will.
- mabrungard
- Piss Artist
- Posts: 250
- Joined: Sat Dec 15, 2012 3:17 pm
- Location: Indianapolis, Indiana
Re: Bedford water
That tap water already has a nice amount of chloride and sulfate for brewing bitters and pales. Adding more chloride and sulfate could easily cause 'minerally' flavor in the finished beers. High sulfate in conjunction with chloride over 100 ppm is a recipe for minerally taste. Since the tap water already has over 100 ppm Cl, you wouldn't want to add any more. Using CRS for neutralizing alkalinity in this water is not prudent. I suggest moving to a cleaner acid such as phosphoric or lactic since they add no chloride or sulfate.
But this is not to say that you shouldn't add more sulfate for those hop-focused beers. I just recommend that you add that sulfate via gypsum instead of that CRS acid blend.
But this is not to say that you shouldn't add more sulfate for those hop-focused beers. I just recommend that you add that sulfate via gypsum instead of that CRS acid blend.
Martin B
Indianapolis, Indiana
BJCP National Judge
Foam Blowers of Indiana (FBI)
Brewing Water Information at: https://www.brunwater.com/
Like Bru'n Water on Facebook for occasional discussions on brewing water and Bru'n Water
https://www.facebook.com/pages/Brun-Wat ... =bookmarks
Indianapolis, Indiana
BJCP National Judge
Foam Blowers of Indiana (FBI)
Brewing Water Information at: https://www.brunwater.com/
Like Bru'n Water on Facebook for occasional discussions on brewing water and Bru'n Water
https://www.facebook.com/pages/Brun-Wat ... =bookmarks
Re: Bedford water
Sounds interesting but how would i determine how much phosphuric acid to use?
- Eric
- Even further under the Table
- Posts: 2919
- Joined: Fri Mar 13, 2009 1:18 am
- Location: Sunderland.
Re: Bedford water
Using your Salifert kit you will be able to measure the acid's influence on your water and hence the amount required and believe me, it's worth the effort getting the residual alkalinity right.timbobist wrote:Sounds interesting but how would i determine how much phosphuric acid to use?
Not trying to be smart, it does depend how concentrated the acid you buy is, which if very concentrated you might then dilute to a level practical for safety, storage and easy accurate measurement in use.
Phosphoric acid is not without its own problems. Only tried lactic acid once and that I could taste, but that was twenty odd years ago when you could buy it in Boots.
Without patience, life becomes difficult and the sooner it's finished, the better.
- Aleman
- It's definitely Lock In Time
- Posts: 6132
- Joined: Sun Jun 03, 2007 11:56 am
- Location: Mashing In Blackpool, Lancashire, UK
Re: Bedford water
I agree with Eric, I can taste lactic in even quite small quantities . . . phosphoric isn't taste neutral either 
I tend to use Sulphuric or Hydrochloric depending on the results I want to achieve with the water and recipe I am using.
I tend to use Sulphuric or Hydrochloric depending on the results I want to achieve with the water and recipe I am using.
- Eric
- Even further under the Table
- Posts: 2919
- Joined: Fri Mar 13, 2009 1:18 am
- Location: Sunderland.
Re: Bedford water
Oscar Wilde was famous for saying he could resist anything but temptation, so how could I desist to extemporise this theme after returning from my local hostelry to find Aleman agreeing with a comment of mine? Moderators, please if you might, treat this with some comPASSION for the following as is writ, is done with PASSION.
There was once a saying in my home town by those with conviction of belief or cause that if what they claimed wasn't true, they would "Show their arse in Binns's window". Binns was then the most prestigeous shop with the largest window displays in Sunderland (home of several breweries of that era) that would be later gobbled up by House of Fraser and subsequently closed when taken by Al Fayed. If you knew Sunderland's main street, you would comprehend the humility offered by by such a pledge.
My favourite local can't be that bad, there was a sign to greet us saying Jennings' head brewer will be in attendance next Wednesday. I can't remember all consumed this evening, specifically the first, which, if any were, it might have suffered from excessive mineralisation, but was that so it might just have been sulphate rather than chloride to my taste. The last pint was Ringwood's Old Thumper, so you might have some idea of the session, but midway we had a pint of Hobgoblin by Wychwood and while sipping that I was reminded of this thread and thought if that was made with less than 100 ppm chloride and 100 ppm calcium, I'd show my arse in Binns's window. Are such beers known of in America?
There was once a saying in my home town by those with conviction of belief or cause that if what they claimed wasn't true, they would "Show their arse in Binns's window". Binns was then the most prestigeous shop with the largest window displays in Sunderland (home of several breweries of that era) that would be later gobbled up by House of Fraser and subsequently closed when taken by Al Fayed. If you knew Sunderland's main street, you would comprehend the humility offered by by such a pledge.
My favourite local can't be that bad, there was a sign to greet us saying Jennings' head brewer will be in attendance next Wednesday. I can't remember all consumed this evening, specifically the first, which, if any were, it might have suffered from excessive mineralisation, but was that so it might just have been sulphate rather than chloride to my taste. The last pint was Ringwood's Old Thumper, so you might have some idea of the session, but midway we had a pint of Hobgoblin by Wychwood and while sipping that I was reminded of this thread and thought if that was made with less than 100 ppm chloride and 100 ppm calcium, I'd show my arse in Binns's window. Are such beers known of in America?
Without patience, life becomes difficult and the sooner it's finished, the better.

