(...Continued!)
Erm ... are you ready for this? It's an excellent example of me documenting stuff to the nth degree because I can't rely on my mangled head to retain very much. So, if I've got the work done anyway, I may as well make it available to all of you! Some are going to find it revealing. Will that include you?
Prologue: ... A what! And can you have a "Prologue" on a post that starts "continued"? I began this "digression" as a proof-of-concept of the water addition calculator I was attempting to build. I knew my next step was to take a while and thought to fill in some stuff to hold any interest meantime. It's turned into a stand-alone post using Bru'n Water anyone can follow (now!), and that can probably be copied on any water calculator. It is using the battle between "alkalinity" and "hardness" to get a suitable mash configuration. It is using the misuse of mixing units (explicit weight, like in grams, and concentration values, like in "mg/L" pr "ppm"). It is using "normalization" to fix this misuse, a posh name for a very straight-forward process (in this case). And it is so simple others must have come across it? But I've never found a description: So here it is! (So simple I am arrogant enough to think I can write a water addition calculator. But it'll take me a little while, so meantime):
Apologies for the time take getting this out. I was convinced I could get Bru'n Water to work with
explicit additions of water treatment salts as well as the usual "water profile". And I was getting in a right mess trying to do it. It doesn't, I don't think any water calculator can, But I wasted lots of time trying. I was also doubtful about using two copies of a water calculator to do the working out (or two "iterations"). Too messy? Too complicated? But turns out to be easy enough, and many magnitudes less complicated than trying to get one copy of the calculator doing the job. Water calculators rely heavily on "concentration" values (grams-per-litre, ppm, etc.) ... trying to make them use explicit values was more of a task than I'd anticipated!
I couldn't be bothered trying to modify that last example
recipe ... that's been and (not very well) done. I'll use the following example which is to be brewed next (the grain is already weighed out). It too is an "X-Ale", from 1914 (start of WWI), dug out by Ron Pattinson from the Fuller's records (1914 Fullers X Ale from his "Armistice!" book). The Fuller's recipe differs in being a more "traditional recipe" (for that time) using maize and no crystal or roast malts like in the Courage recipe. Both use "No.3 Invert Sugar" to add a bit of colour (but still "light" in colour by late 20th C. "Mild Ale" standards).
.

- Fullers 1914 X orig iv.jpg (479.15 KiB) Viewed 3155 times
.
The first illustration is the recipe with water treatment salts calculated in the "usual" manner. As with the earlier Courage recipe the calculated Calcium addition is large and so is the "Alkalinity" addition to compensate. You should be able to take from this illustration that it's quite feasible to mix "grams" with "mg/L" ("ppm") if careful, but significant distortion
will be present if not (very!) careful. What the calculator needs to make things a whole lot easier, is a handful of "adaptions". This is my selection of "adaptions" that will do the job. First step is making a copy of the calculator with all the original recipe's bits and bobs intact (most "water calculators" are spreadsheets, so simply copy the file and open that). I'm using "Bru'n Water" in this example, but other calculators may be suitable.
On the copy go to the "Water Adjustment" page (shown in the illustration above) and zero seven (leave the one for "Calcium Chloride") values under "Addition (gram/L)".
Next select "Water Volume" under "Mash", "Total Water Additions", and enter one half of the "Total Batch Volume".
Under "Sparge, "Total Water Additions", enter "zero". We are only calculating Mash additions and want no distractions.
Add together the analysis for Calcium, and Magnesium-divided-by-two, that's in the source water. They will be "concentration values", e.g. "PPM" or "mg/L". Only use half the value given for Magnesium as it less impact than Calcium and "half" is a very common assumption.
Decide on a base "concentration value" for Calcium ions in the Mash. This value probably shouldn't be less than 35PPM (or mg/L). Values of 35, 50 or even 70 should be "safe". The actual value isn't important, but it's best to stay within the limits stated (35-70PPM). I picked 70.
Subtract the value calculated above for Calcium and Magnesium from the "base concentration value" (35, 50, or whatever) for Calcium ions. Under the "Additions" column of "Water Additions" find "Calcium Chloride" and start entering values until that modified "base concentration value" appears in the adjacent "Calcium (ppm)" field. A lot of phaff, but easier than trying to do the sums in your head or on scraps of paper.
At this point the displayed pH is not valid. The last point ("... not valid") is important, it's always catching me out, so I'm forever thinking it's wrong!
The "Normalized" base value for Calcium Chloride is displayed under "Mash", "Total Mineral Additions". At this moment, that value along with a note of the "Calcium (ppm)" value (the modified "base concentration value" calculated above) is the only useful information to be taken from the spreadsheet.
.

- Fullers 1914 X adap iv.jpg (124.28 KiB) Viewed 3155 times
.
It's a bit of a cheat in that I'm using Martin Brungard's grafting (via his "Bru'n Water" calculator) to do the hard work. This technique is so kookie I had to spend another day or two "rediscovering" what I'd done (so it's "double-checked!), and a few days later, seems I forgot it all and had to repeat the "rediscovery" steps again (so it's "triple-checked!). That was down to not appreciating most values get temporarily invalidated by these actions. Take note! I've mentioned any above where possible. And I'm still expecting to uncover a "killer" mistake I've missed in allowing this all to happen (haven't found it yet!). I make no apology for the contortions; it wasn't me who mixed up all these concentration and explicit measures!
In the following illustration the "Water Volume" for "Mash" is corrected to reflect the real amount. This value is not changed if using the "recommended" 1/2 x "Batch Volume". But the "recommended" 2/3 x "Batch Volume" might be used? Any value one or less used (perhaps not advisable to go below 1/2). Or, as in this case, the "full-boil-volume" ("no-sparge") is used which includes all the non-recoverable losses calculated throughout the remaining brew stages (Grain Absorption, Hop Absorption, Boil-off, etc.). In any case:
The "normalized" amount of Calcium is the temporary "grams per litre" concentration value for a specific amount of water, converted to the
actual amount for
any amount of water measured out, adding that amount (in grams, or in the examples case, millilitres) directly to the mash water. Note that for any review of usage, the "concentration" amounts remain the same, only the "explicit amount" needs to be recalculated for any change in mash/sparge water quantity, "hardness" (calcium and magnesium) and alkalinity salts.
NOTE: To calculate an "explicit" amount of Calcium salt I've chosen a 33% solution Calcium Chloride, hence the extra-large amounts ... Bru'n Water continues to display grams for liquid CaCl
2 instead of remembering to switch to millilitres (but it means millilitres, not grams). Anyhow, I find liquid much easier to deal with. Bru'n Water can be switched between using liquid, anhydrous or dihydrate but won't alter any values entered. "Anhydrous" is the 33% liquid value multiplied by 33/100. "Dihydrate" is the anhydrous number times 1.292.
Lower the "Estimated Mash pH" to match the original with acid (small changes can be made by altering the "base concentration value" for Calcium - see above - but acid alterations are easier). If the pH needed to be raised "alkalinity" salt would be added.
.
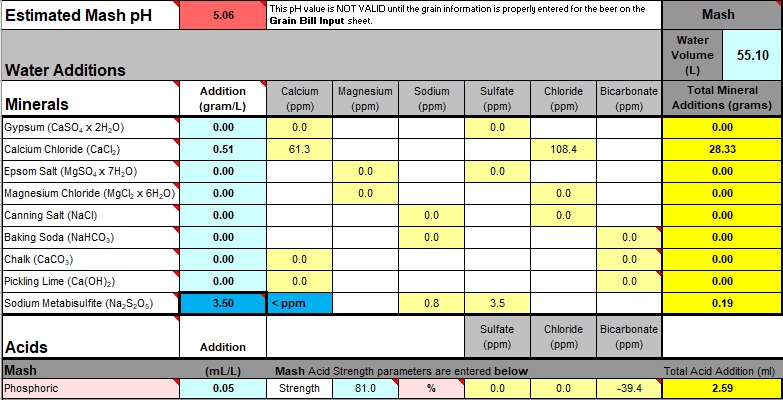
- Fullers 1914 X norm iv.jpg (124.26 KiB) Viewed 3155 times
.
Returning to the original spreadsheet:
Toggle the "Add Hardness Minerals to Kettle?" option to "Yes". Not obviously necessary for a "no-sparge" recipe but do it anyway or Bru'n Water may miscalculate pH estimate (in this case the estimate may not be correct anyway, but it won't look so bad!).
Amongst the "Water Additions" is "Calcium Chloride". This includes the 28ml of 33% CaCl2 solution added as a "Normalized" amount to the mash. Make sure this amount is subtracted from the calculated amount to be added "post-mash". There is no way to remove this amount in the calculator in such a selective manner. The next best available method was to place a note in the Calculator's "Batch Notes".
A note should also be added to the Calculator's "Batch Notes" reminding the user that the displayed "pH", the "Mashing Water Profile" and the "Finished Water Adjustment" lines are also invalidated by the procedures that allow "normalized" values to be used.
Minor adjustment
can be made to the salts to get everything right again.
.
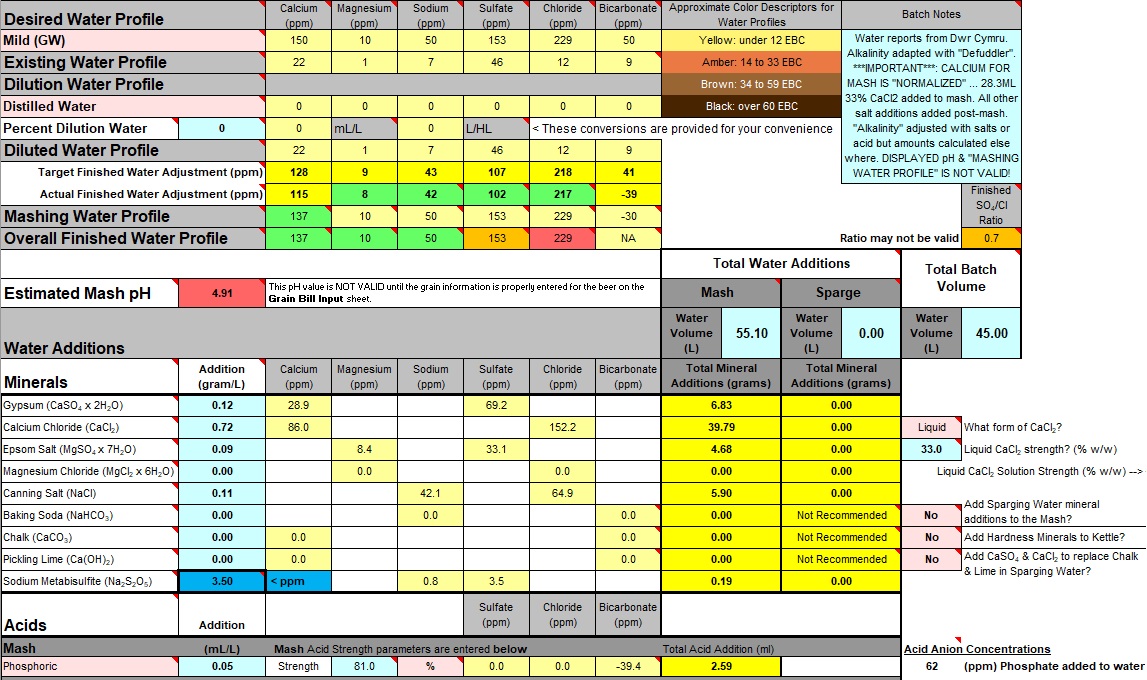
- Fullers 1914 X fini iv.jpg (328.78 KiB) Viewed 3155 times
.
Epilogue: ... Well, if I've got a Prologue, I'm not going to miss out on having an "Epilogue".
Trying to follow all that without having an actual example in front of you would be asking too much. This is really "twisting the arm" of Bru'n Water to make it reluctantly comply with "explicit measures. This was a "reference" post. The benefits of working this way are only likely to be obvious in practice. Ironically, the biggest benefit is simplicity! But we are so used to the job as it is, which it has to do that way to work across a complete range of scales (and we are very much at the small end of scale), we overlook the baffling complexity it introduces. So, sure, it's going to be complicated to untangle it.
Trying to get an existing "concentration" biased calculator to do the job has been a nightmare. If you're not keen, don't do it! I'll come up with something easier (one day?)
And subjects this post just touches on but steers you away from: Measuring salts to hundredths of a gram accuracy ... yet only applying the amounts to a random proportion of the beer ("concentration" amounts are often specified to duplicate a certain profile, but unless told otherwise and provided with the necessary additional data. a "concentration amount" must be applied to all water in the process). Salts added to the mash that only affect the mash are targeted at grain amounts, not water, and therefore are best added as "explicit" amounts, or else you add quantities well in excess of the requirement just to ensure you don't underdose. The small volumes used in homebrewing makes "explicit" amounts well within the ability of the brewer. Yet the same small quantities mean the errors induced by imprecise management of "concentration" amounts are magnitudes greater than in commercial practices.

